
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product.
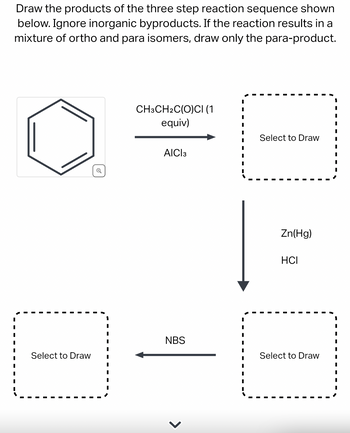
Transcribed Image Text:Draw the products of the three step reaction sequence shown
below. Ignore inorganic byproducts. If the reaction results in a
mixture of ortho and para isomers, draw only the para-product.
Select to Draw
Q
1
I
I
CH3CH2C(O)CI (1
equiv)
AICI 3
NBS
>
Select to Draw
Zn(Hg)
HCI
Select to Draw
I
I
I
I
I
I
I
I
I
1
I
I
I
I
I
I
I
I
I
Expert Solution
arrow_forward
Step 1: Introduction
Benzene undergoes acylation on treatment with acyl chloride in the presence of Lewis acid. This reaction is called Friedel-Crafts acylation.
The second reaction is reduction of carbonyl group.
NBS is a brominating agent and is used for bromination at allylic and Benzylic positions.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following reactions in sequential order. Show the product after each step and put a box around the final product. (Show intermediate structure after each reagent)arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. 1. CH3MgBr (excess) 2. H₂O CI Qarrow_forwardProvide the product(s) for the following reaction. If no reaction, draw the starting materials.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. H3O*arrow_forwardShow how to synthesize the following product as the major product starting with 2,2-dimethylpropane as the starting material. You may use additional reagents and any number of steps. Be sure to list each step with all reactants/reagents/conditions required. (Do not use hydrogenation reactions). Write the process.arrow_forwardDraw the major product of this reaction. Ignore byproducts.arrow_forward
- Acetal product formation requires an acid catalyst in the second step, but NOT in the first step. True or False?arrow_forwardDraw the major product of this reaction. Use wedges and dashes to indicate stereochemistry where applicable. Ignore inorganic byproducts. HO OH OH OH OH Q TSOH Drawingarrow_forwardAn ether can be prepared from an alkene and an alcohol by electrophilic addition, as shown in the reaction below. H+ CH₂CH₂OH Complete the mechanism for this reaction by adding curved arrows and products. Add steps as necessary, and be sure to include lone pairs and charges where relevant.arrow_forward
- Draw the major products of this reaction. Ignore stereochemistry. Ignore inorganic byproducts. 1. KMnO4, H₂O* 2. NalO4 3. Na2S2O3, H₂Oarrow_forwardDraw the products of the three step reaction sequence shown below. Ignore inorganic byproducts. If the reaction results in a mixture of ortho and para isomers, draw only the para-product. Select to Draw CH3CH2C(O)CI (1 equiv) AICI 3 HNO3 (1 equiv) cat. H2SO4 > Select to Draw NH2NH2, KOH heat Select to Drawarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting structure, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. be sure to account for all bond-breaking and bond-making steps. then draw any missing organic intermediates or products for this reaction. include all lone pairs in the structures. ignore inorganic byproducts, counterions, and solvents.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY