
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
In the lowest energy conformation of the compound below, how many alkyl substituents are equatorial?
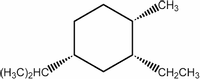
Transcribed Image Text:The image shows the structural formula of a substituted cyclohexane molecule. The cyclohexane ring, a hexagon, is at the center of the structure.
- The upper right carbon atom in the cyclohexane ring is bonded to a methyl group (CH₃), represented with a wedge to indicate it is positioned above the plane of the cyclohexane ring.
- The lower right carbon atom is bonded to an ethyl group (CH₂CH₃), shown with a dashed wedge indicating it is positioned below the plane of the ring.
- The lower left carbon atom is bonded to an isopropyl group ((CH₃)₂HC), also represented with a dashed wedge indicating it is positioned below the plane of the ring.
This diagram illustrates the stereochemistry of three substituents on the cyclohexane ring, using wedges and dashed wedges to indicate the orientation of the groups relative to the plane of the ring.
![**Question 37:**
In the lowest energy conformation of the compound below, how many alkyl substituents are axial?
[Diagram of a cyclohexane ring with three substituents]:
- One methyl group (CH₃) attached with a wedge.
- One ethyl group (CH₂CH₃) attached with a dash.
- Another ethyl group ((H₃C)₂HC) attached with a dash.
**Answer Choices:**
A) 0
**Explanation of Diagram:**
The chemical structure depicted in the image is a cyclohexane ring. It is illustrated with three substituents:
1. A methyl group (CH₃) is attached to one carbon of the cyclohexane using a wedge, indicating that it is pointing above the plane of the ring.
2. Two ethyl groups ((H₃C)₂HC and CH₂CH₃) are attached to different carbons via dashed lines, indicating they are pointing below the plane of the ring.
In the context of cyclohexane conformations, wedges typically indicate groups in the equatorial orientation, while dashes can either be equatorial or axial depending on the ring flipping. The question asks for the number of axial alkyl substituents in the most stable chair conformation.
Given the setup, with all substituents in the equatorial orientation in the lowest energy conformation, there are 0 axial alkyl substituents. This makes option A) 0, the correct answer.](https://content.bartleby.com/qna-images/question/d6133fd2-0efc-455d-9539-fd38cdd74c86/131362d4-4102-4a7e-a17e-38b34797f5ef/rq6xpn5_thumbnail.png)
Transcribed Image Text:**Question 37:**
In the lowest energy conformation of the compound below, how many alkyl substituents are axial?
[Diagram of a cyclohexane ring with three substituents]:
- One methyl group (CH₃) attached with a wedge.
- One ethyl group (CH₂CH₃) attached with a dash.
- Another ethyl group ((H₃C)₂HC) attached with a dash.
**Answer Choices:**
A) 0
**Explanation of Diagram:**
The chemical structure depicted in the image is a cyclohexane ring. It is illustrated with three substituents:
1. A methyl group (CH₃) is attached to one carbon of the cyclohexane using a wedge, indicating that it is pointing above the plane of the ring.
2. Two ethyl groups ((H₃C)₂HC and CH₂CH₃) are attached to different carbons via dashed lines, indicating they are pointing below the plane of the ring.
In the context of cyclohexane conformations, wedges typically indicate groups in the equatorial orientation, while dashes can either be equatorial or axial depending on the ring flipping. The question asks for the number of axial alkyl substituents in the most stable chair conformation.
Given the setup, with all substituents in the equatorial orientation in the lowest energy conformation, there are 0 axial alkyl substituents. This makes option A) 0, the correct answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- translate the bond-line notation structure to the Newman projection by filling int the missing groups (A, B, C, D or E) on the lines in the Newman projection so they match the conformation given in the original structure. Circle if the conformation is a staggered or eclipsed.arrow_forwardtranslate the bond-line notation structure to the Newman projection by filling int the missing groups (A, B, C, D or E) on the lines in the Newman projection so they match the conformation given in the original structure. Circle if the conformation is a staggered or eclipsed.arrow_forwardPlease don't provide handwritten solutionarrow_forward
- Q2. Answer any TWO of the following parts: (a) Draw the two main conformations that exist for cyclohexane. Explain clearly why one conformer is more stable than the other. Using cis-1-ethyl-3-methylcyclohexane, as an example, explain how ring flipping occurs. Draw both conformers of cis-1-ethyl-3-methylcyclohexane and explain clearly which one predominates. (b) What is polarimetry? The specific rotation of (R)-carvone is - 61°. A chemist prepared a 750 mg mixture of (R)-carvone and its enantiomer in 10 ml of ethanol and placed the solution in a 10 cm polarimeter cell. The observed rotation was - 4.125°. Calculate the specific rotation for the above mixture. What is meant by enantiomeric excess? Then determine the % enantiomeric excess (% ee) in the mixture. (i) (ii) (iii) What % of the mixture is (R)-carvone and (S)-carvone?arrow_forwardDraw the most stable conformation of the molecule shown below. The most stable conformation is the one in whicharrow_forward2arrow_forward
- Which of the following is the correct Newman projection for the following compound as viewed down the indicat conformation shown? CO II O III O IV OV CH3 CH3 CH31 CH3 CI || Br Br H CH3 CH3 ||| Br CH3 CH3 IV CI Br CH3arrow_forwardFont Paragraph Using a Newman projection, draw the most stable conformation of CH2-CH2. | ОН ОНarrow_forwardIn the most stable chair conformation of the cyclohexane below, how many methyl groups would be axial? CH3 CH3 three zero two all four CH3 CH3arrow_forward
- Please show reaekson and don't use hend raitingarrow_forwardWhen you obtained a geometry optimized structure of methylcyclopentane, did you determine the energy of the most stable conformation? Why or why not?arrow_forwardWhich of the following is an accurate conformational drawing for compound Z? Zarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY