
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
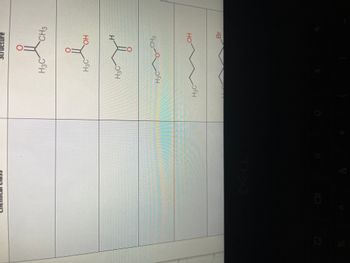
Transcribed Image Text:%
8
*
H3C
Structure
новою
CH3
привет
ОН
H
нсум
H3CO-CH3
НО
Br
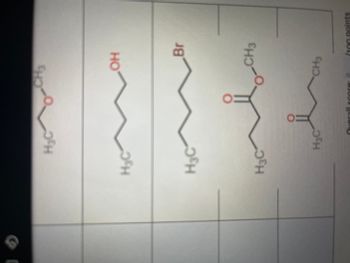
Transcribed Image Text:H₂O
H₂C
30
H3C
H3C
ене
OH
Br
CH3
CH3
(109 points
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H3C Imm H3C y Br CH3 Br (CH₂ CH₂)3N NaOCH₂CH₂ CH₂CH₂OHarrow_forwardWhich fatty acid would you expect to be have the lowest melting point? # 3 80 F3 71 $ 4 A) CH,CH=CH(CH,), CH=CHCOOH B) CH₂(CH₂),COOH C) CH₂(CH₂),COOH D) CH₂CH=CH(CH₂),COOH E) CH₂(CH₂) ₁4COOH Q F4 dº L % 5 Question 6 of 19 F5 14 <6 F6 & 7 ▷▷ F7 * 0 8 DII F8 9 DD F9 0 7 F10arrow_forwardAn isomer of CH3CH=CHCH2_OH isarrow_forward
- Take a look at this molecule, and then answer the questions in the table below it.arrow_forwardDraw the product Et2Cuarrow_forwardArrange the following radicals in order of increasing stability (least stable to most stable). 4th attempt Feedback Question List (4 images) (Drag and drop into the appropriate area) No more items 3 OF 25 QUESTIONS COMPLETEDarrow_forward
- An isomer of CH3CH=CHCH2-OH isarrow_forwardesc ! 1 Take a look at this molecule, and then answer the questions in the table below it. CH₂OH OH Q H H OH H O Explanation H OH 2 H ▼ OH H O Is this a reducing sugar? CH₂ H OH H W O Does this molecule contain a glycosidic bond? H If you said this molecule does contain a glycosidic bond, write the symbol describing it. Check OH If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. OH H # 3 E $ 4 R % 5 T A MacBook Pro 6 O yes O no O yes O no 0-0 - 0 V & 7 a В X * ローロ © 2023 McGraw Hill LLC. All Rights F 8 3arrow_forwardExplain Chlorination: CH3CH2CH3 + Cl2arrow_forward
- NH2 ČH3 CH3 NH2arrow_forwardCould you please solve these two and also this question below? which of the following is a disaccharide? a. Fructose b. Galactose c. Maltose d. Amylose e. Glucosearrow_forwardTriglycerides can be classified by the number of double bonds present in the hydrocarbon chain. Categorize this triglyceride based on the degree of saturation 04-0-8-compon C-(CH₂)-CH₂ CH-0-C-(CH₂CH=CH-CH=CH-CH₂ O=U C46-0-8 -10M) CH, CH= CH₂-0-C H=CH-CH=CH-CH=CH-CH₂ O Polysaturated Saturated Monounsaturated O Polyunsaturatedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY