
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
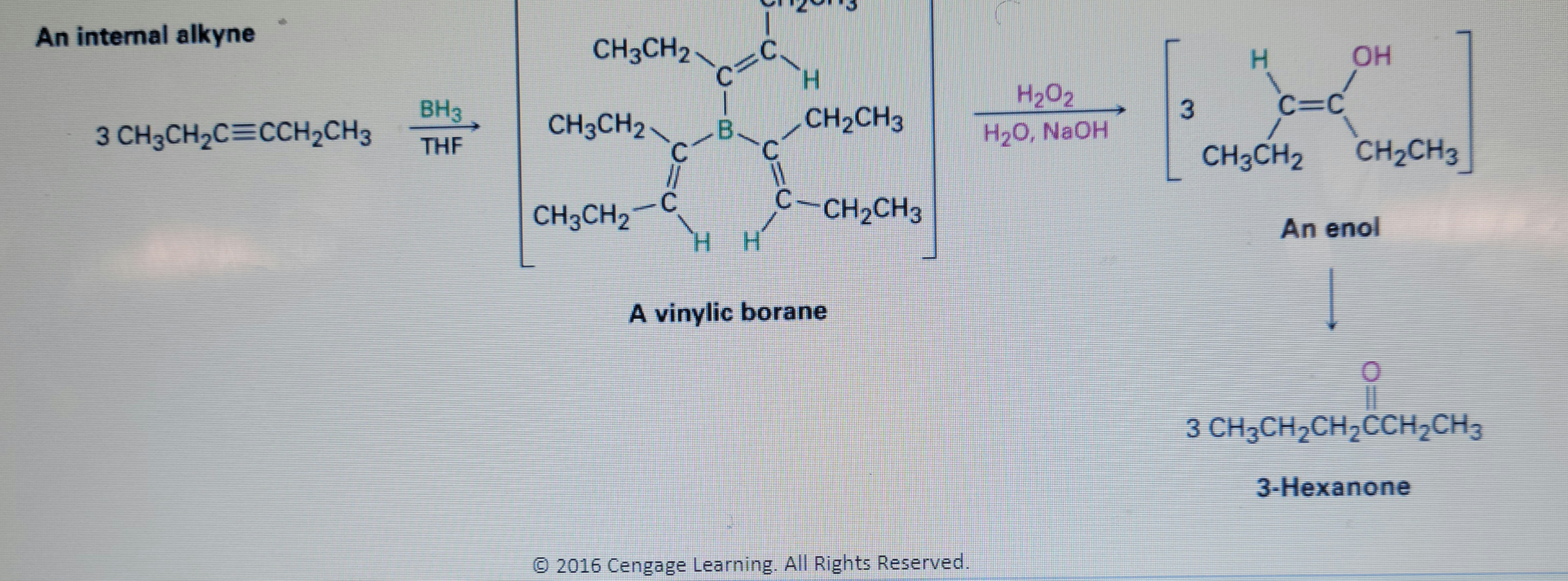
Transcribed Image Text:An internal alkyne
CH3CH2
H.
OH
H.
BH3
H202
3
C=C
CH3CH2
B.
CH2CH3
3 CH3CH2C=CCH2CH3
H20, NaOH
THF
C.
C.
CH3CH2
CH2CH3
-C
CH3CH2
C-CH2CH3
HH
An enol
A vinylic borane
3 CH3CH2CH2CCH2CH3
3-Hexanone
© 2016 Cengage Learning. All Rights Reserved.
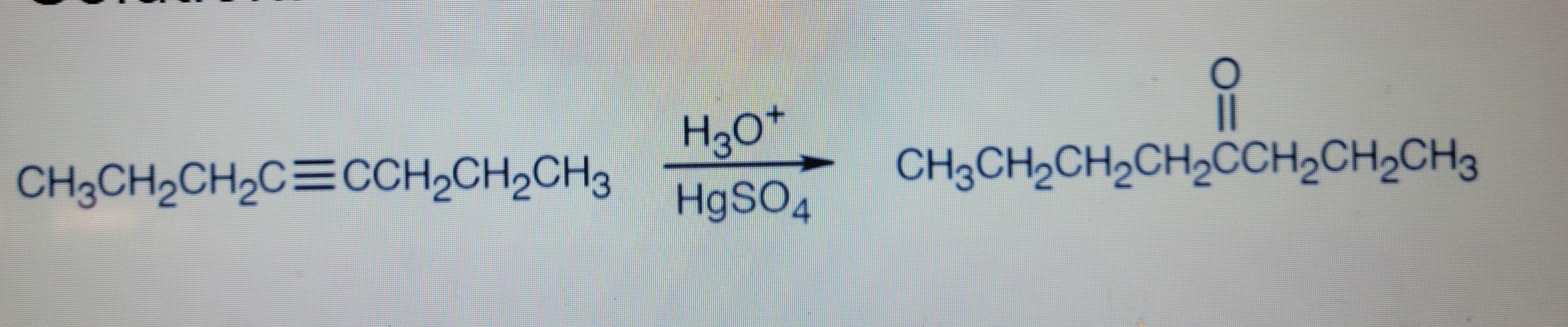
Transcribed Image Text:H30*
CH3CH2CH2C=CCH2CH,CH3
CH3CH2CH2CH2CCH2CH2CH3
H9SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the organic product: O O A. IV OB. III OC. I OD. II I + AlCl3 C1 ? II III IV OHarrow_forwardWhat are the products of each alkene addition reaction? a. CH;-CH-CH=CH2 + Br2 CH3 b. CH,=CH-CH;+ Cl, CH3 c. CH-Ç-CH=CH2 + HCl ČH3 CH3 d. CH;-CH-CH=C-CH3 + HBr CH;-CH,arrow_forwardOrganic chemistryarrow_forward
- 8c) What is the relation between the following pairs H₂C O a. Cis-Trans O b. Structural O c. not isomers O d. Identical H3C CH3 H₂CH.arrow_forward45. What is the major organic product? H. (өxсess) C d. N. HO N- CIarrow_forward7. kot The single bond between the carbonyl carbon and oxygen in the group. а. carboxyl Ob. carboxylate ion C. carboxylic acid Od. carboxylic acid anhydride e. carboxylic ester Of. condensation polymerization g. dimer h. ester i. esterification j. ester linkage Ok. fatty acid O1. saponificationarrow_forward
- What term describes the structural relationship between (2R,3R,4S)-2,3,4-trichloroheptane and (2R,3R,4R)-2,3,4-trichloroheptane? A. enantiomers B. diastereomers OO OO C. constitutional isomers D. not isomersarrow_forwardOrganic Chemistry.The section asks for the synthesis: draw/ provide the likely organic productsarrow_forwardPlease provide structures for the given reactions NN, OO, and PParrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY