
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw all the reagents and solvents necessary to conduct the following reaction.
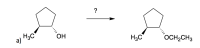
Transcribed Image Text:### Educational Content: Organic Chemistry Reaction
#### Starting Material:
- The structure on the left-hand side is 1-methylcyclopentanol.
- It consists of a five-membered carbon ring (cyclopentane).
- Attached to the ring are:
- A methyl group (CH₃) bonded to the first carbon.
- A hydroxyl group (OH) bonded to the same first carbon but projecting in the opposite direction (indicated by the dashed bond).
#### Reaction Scheme:
- An arrow pointing from the starting material to the product represents a chemical reaction.
- The question mark above the arrow indicates that the reagent/environment needed for the transformation is unknown or needs to be determined.
#### Product:
- The structure on the right-hand side is 1-methoxyethylcyclopentane.
- It is similar to the starting material with the same five-membered carbon ring (cyclopentane).
- Attached to the ring are:
- A methyl group (CH₃) bonded to the first carbon.
- A methoxyethyl group (OCH₂CH₃) replacing the hydroxyl group at the same carbon, in the opposite direction (indicated by the dashed bond).
### Explanation:
- The structure suggests that the hydroxyl group (OH) in 1-methylcyclopentanol is being transformed into a methoxyethyl group (OCH₂CH₃).
- This reaction typically involves an etherification process where the hydroxyl group is substituted with an ethoxy group through a specific reagent or condition.
### Possible Reaction Mechanisms:
- **Williamson Ether Synthesis**: An alkoxide ion reacts with an alkyl halide.
- **Acid-Catalyzed Etherification**: An alcohol reacts with an unsaturated compound in the presence of an acid catalyst.
Understanding the detailed mechanism for such a transformation is vital for students studying organic synthesis and reaction pathways.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Understanding Organic Chemistry Workbook 3 H,C-CH3 bb H3C-O-C-CH2-C-CH2-CH3 Туре of functional group H,C-CH3 || H3C-0-C-CH2-C-CH2-CH3 IUPAC H2C-CH3 H,C-o-6-CH,-C-CH,-CH, Common Name CH3 9 H3C-CH-C-CI c. Туре of functional group CH3 9 H3C-CH-C-CI IUPAC CH3 O H3C-CH-Ö-CI Common Namearrow_forward3. (Chapter 11) Write the IUPAC name and then the common name for the following: CH3 || H3C-CH-CH2-CH2- C-H IUPAC CH3 || Hас — сн— сн, — Сн,—с—н Common Name Understanding Organic Chemistry Workbook 4 4. (Chapter 11) Write the IUPAC name and then the common name for the following: of H,C-CH, K* IUPAC H;C-CH,- -o K* Common Namearrow_forward2. Consider the following organic molecules A-H. Many of them are natural products isolated from Nature. Please answer the following questions by writing the Letter pertaining to each molecule next to the question. -N- H;C "CO,H N. Но CH2 ČH3 B strychnine (a plant alkaloid) hirsutic acid A nepetalactone (essence of cutnip) (a fimgul metabolito) H- H. I CH,0 E helminthosporal (a imgal toxu) D progesterone F cantharidin (a progestin hommone) (am insect vesicant) Br NO2 Br CI G untenine C (E)-1,6-dibromo-2,7-dichloro-3,7-dimethyloct-3-ene (fom red algae) (4 sponge metabolite)arrow_forward
- MISSED THIS? Read Section 3.9 (Pages 113-118); Watch IWE 3.16. Part A How many fluorine atoms are present in 5.56 g of CF ? Express your answer to three significant figures. ► Vlew Available Hint(s) atoms Submit Provide Feedback JAN 31arrow_forwardChoose the correct answer of organic chemistry questionsarrow_forwardA pair of electrons(s) in the s bond spreads out into empty p orbital, stabilizing electron-deficient carbon atom, the process is known as ________. a) Inductive effect, b) Resonance, c) Hyperconjugation. The carbon atom in dibromocarbene has only ______ electrons in its valence shell with _____ hybridized and ________ geometry. a) Four, sp3, trigonal, b) Five, sp, planar, c) Six, sp2, trigonal, d) Five, sp3, planar.arrow_forward
- Need help drawing it on this chartarrow_forwardHow do I draw this resonance structure?arrow_forwardMembers of this series are not as chemcially reactive as the other hydrocarbons, and each has a higher molecular weight as a result of an additional -CH_2_. (That's suppose to be a small two) A) hydrocarbon B) polymer C) alcohol D) paraffin Can you help me understand which one it is please?!arrow_forward
- Please correct answer and don't use hend raitingarrow_forwardWhich line (A or B) corresponds to the energy change for an ortho/para substitution?arrow_forwardHere is the chemical structure of methyl propanamide: H H. H H. H-C- C-C -N-C-H H. Decide whether each molecule in the table below is another mnolecule of methyl propanamide, a molecule of an isomer of methyl propanamide, or a molecule of an entirely different compound. molecule relationship to methyl propanamide NH-CH, (Choose one) another molecaule of methyl propanamide a molecule of an isomer of methyl propanamide a molecule of a different compound CH,-CH,-C=0 H H :o: H (Choose one) H-C H- H :0: H H H C-C (Choose one) H. H 回 山回arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning