
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
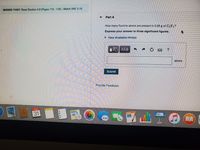
Transcribed Image Text:MISSED THIS? Read Section 3.9 (Pages 113-118); Watch IWE 3.16.
Part A
How many fluorine atoms are present in 5.56 g of CF ?
Express your answer to three significant figures.
► Vlew Available Hint(s)
atoms
Submit
Provide Feedback
JAN
31
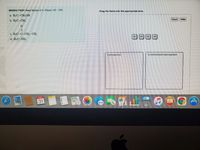
Transcribed Image Text:MISSED THIS? Read Section 3.11 (Pages 123 - 126).
Drag the items into the appropriate bins.
a. H3C-CH, OH
Reset
Help
b. H3C-CH3
c. H3C-C-CH,-CH3
(a)
(b)
(e)
(d)
d. H C-NH,
hydrocarbon
functionalized hydrocarbon
JAN
31
Expert Solution
arrow_forward
Step 1
“Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved, then please specify the question number or post only that question.”
According to the mole concept, in terms of mass, the amount of substance in moles is equal to the ratio of its mass to the molar mass.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A beaker contains 100.0 mL of water. To it is added 10.0 mL of dilute nitric acid (HNO3). Calculate the total moles of water in the beaker after the acid is added. (Assume the density of the nitric acid is the same as that of water.) Density of water= 1.00 g/mLarrow_forwardVitamin C, an antioxidant present in citrus fruits, contains only C, H, and O. Combustion of 10.0 g of vitamin C produces 14.99 g of CO2 and 4.092 g of H2O. Determine the molar mass of the compound if it is between 150.0 and 220.0 g/mol. Provide an answer to four significant figures.arrow_forwardWhat is the mass, in grams, of 7.30×1020 molecules of caffeine, C8H10N4O2? Express your answer using three significant figures.arrow_forward
- How many C atoms are present in 8.94 · 1020 CH3C(O)H molecules? Express your answer in scientific notation.arrow_forwardThe chemical formula for hydrogen bromide is HBr. A chemist determined by measurements that 0.030 moles of hydrogen bromide participate in a chemical reaction. Calculate the mass of hydrogen bromide that participates. Round your answer to 2 significant digits. 0 g x10 × Śarrow_forwardThe chemical formula for dimethyl ether is: CH3OCH3 Calculate the molar mass of dimethyl ether. Round your answer to 2 decimal places. 1 g mol x10 ?arrow_forward
- How many atoms of each element (C, H, O) are present in 12.6 g of C12H22O11?arrow_forwardA mixture weighing 30.500 g contained 4.518 g NH4Cl, 15.250 g SiO2, with the remainder being NaCl. Find the percent, by weight, of each component. Show your method for making the calculations.arrow_forwardHow many carbon atoms are in 57.9 g glucose (C6H12O6)? (Provide your answer with the correct number of significant figures, do not include units in your answer) if you answer is 8.79 × 1047 write 8.79E47 where E represents × 10. if you answer is 8.79 × 10-47 write 8.79E-47 where E represents × 10. (Neither 8.79 × 1047 nor 8.79 × 10-47 is the correct answer) Do not include units in your answer.arrow_forward
- How many grams of O2 are needed to form 7.35moles of Fe2O3? 4 Fe + 302 → 2 Fe203 Round your answer to 2 decimal places and, if needed, enter scientific notation like this 6.02e23 (would mean 6.02 x 1023.)arrow_forwardPlease don't provide handwriting solutionarrow_forwardAn empty propane tank weighs 7.22 kg. When it is filled it weighs 25.20 kg. How many propane molecules, C3H8 exist in the tank? Please explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY