
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
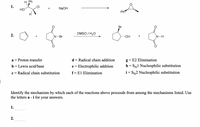
Transcribed Image Text:H Ph
1.
HỒ
NaOH
Ph
Br
DMSO / H20
2.
N-Br
..OH
N-H
Proton transfer
d = Radical chain addition
g = E2 Elimination
a =
b = Lewis acid/base
e = Electrophilic addition
h = SN1 Nucleophilic substitution
I|
c = Radical chain substitution
f= El Elimination
i = SN2 Nucleophilic substitution
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use
the letters a - i for your answers.
1.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 人,。 1. CH,OH Br Br2 HBr ÓCH, 2. CH2 LiT a = Proton transfer d = Radical chain addition g = E2 Elimination b = Lewis acid/base e = Electrophilic addition SN1 Nucleophilic substitution h = C = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardX app.101edu.co I UL Week 7: Panonto CH3Br ||| DII Br || 4x X UL LTU 7-1A F4 Rank these alkyl halides in order of decreasing reactivity in an SN2 reaction. 4 Br F5 40 X Question 1 of 24 F6 O C|Chegg.com S F7 A) III < | < || B) || < | < ||| C) ||| < || < | D) | < || < ||| E) | < ||| < || PrtScn X + FB Home F9 A End F10 Tp S # ENG @ 10 PgUp 611 0 Show 10/arrow_forward1c. What is the nucleophile in the following reaction? .Br CH;CO0 Na LO OCCH3 NaBr ld. What is the electrophile in the following reaction? Br CH;COO Na L0OCCH3 NaBr +arrow_forward
- 7. Reaction Scheme. NH₂ NH2 or two differnet methods (no same steps/reagents) C5H12N2 1. xs Mel, xs K2CO3 2. Ag2O, H₂O 3. heat Br2, xs NaOH, xs H₂O OHC 1.03 2. DMS CHOarrow_forward1. H3C H CH3 2. H3C + Br CH3 H3C H excess NH3 NH4CI H H NH2 C2H5 N-C₂H5 C2H5 + C2H5 H-N-C2H5 Br C2H5 a Proton transfer b Lewis acid/base e Electrophilic addition h = Syl Nucleophilic substitution f El Elimination i = SN2 Nucleophilic substitution c Radical chain substitution g E2 Elimination j Electrophilic aromatic substitution d Radical chain addition Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers.arrow_forward[Review Topics] [References] HO pyridine 1. SOCIl, + sO2 + H. Aqueous acetone H3C H20 H3C HBr 2. Br H OH optically active racemic f = SN1 Nucleophilic substitution g = SN2 Nucleophilic substitution a = Proton transfer d = E1 Elimination b = Lewis acid/base e = E2 Elimination c = Electrophilic addition The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a - g for your answers. 1. 2.arrow_forward
- What is the major organic product obtained from the following reaction? N J ||| A. B. Z= HN HNH I 1. LiAlH4 2. H3O+ NH₂ C. D. NH₂ NH₂arrow_forwardWhat Is the starting materlal of this reaction? Click on a letter A through D to answer. CH,OH H+ cat. А. С. В. OH D.arrow_forward0 a +H=BC: Where are the curved arrow poshing mechanism as well as the non-bonding electrons. What are the initiation and progation Steps? •What are the two termination Steps for the reaction. B. с A. В. C. D. Brz HV - + • Br: → H-Br: + → + • Br: H-Br: + H + • Br: → H-BC: + •Where are the curved arrows pushing the reaction with bromine atom? What are the radical product including the resonance contributors • Rane the reaction in order of increasing rate. what is the explanation that includes the term Hammond Poštutate? H2 PJ/C HBI Br: 1.BH 3. THE 2. NaOHV/H₂O₂ Bcz (2) hu x 1. What are the major organic productes) of the reaction. Include all Stereochemistry and identify any meso compounds. E.. все hu 2. Would the solution of the products be optically active of not active. what would be the Ceason for the choice?arrow_forward
- Cambridge International AS Level Chemistry ot boe slo plgmes s al snsdismonooli End-of-chapter questions 1 1-bromobutane will undergo reactions when heated, as shown by reactions A and B. ot dwab on s bl auoltse s boaub svsd 20 swoll gab ods-suai la yel snoso sdr ssiganm n2 monl gnivims noiteibar VU luad CH;CH,CH,CH,Br B CH;CH,CH,CH,OH CH;CH,CH=CH, 90u S1s 2O1D Isdh tuo gomia sli ni qu dgid tod a guch pecoue For reactions A and B give the reagents used in each case. b Reaction A was repeated using 1-iodobutane instead of 1-bromobutane. Explain any difference in the rate of reaction observed. p bas- od mon c What type of organic reaction is A? d Show the mechanism for reaction A. e Reaction A was repeated with 2-bromo-2-methylpropane instead of 1-bromobutane. i Name the organic compound formed. elle ii The mechanism of the reaction with 2-bromo-2-methylpropane differs from the mechanism of smitas nosd asd i anol reaction A. Describe how the mechanisms differ. f What type of reaction is…arrow_forwardWhich is the MAJOR product of the following reaction? Et 1) BH3:THF 2) H2O2, NaOH Which of the following best describes a key step in the mechanism for the reaction below? HO ... CH3 -CH3 dihydroxylation + en H3C- H3C- HO. electrophilic addition reaction to form a carbocation intermediate B nucleophilic attack by an alkene to form a cyclic (epoxide) intermediate elimination reaction by abstraction of a beta-hydrogen D free-radical substitution at the carbonyl carbon Which alkene will produce the HIGHEST yield of the alkyl halide below? Br. alkene HBr |arrow_forwardBr tBuO H3C H3C CH2 H2 Alkyl halides undergo nucleophilic substitution and elimination reactions. When the kinetics of the reaction are measured, if the rate of the reaction is found alkyl halide the reaction is second order. The substitution reaction is thus termed SN2, and the elimination reaction is be dependent upon the concentration of the nucleophile as well as the termed E2. These reactions are bimolecular and take place in a single step. In the SN2 reaction, the nucleophile attacks the a-carbon from the backside and displaces the leaving group with an inversion of configuration occurring at the carbon. In the E2 elimination reaction, strong base removes an acidic hydrogen from the B-carbon and an alkene is formed as the leaving group is expelled from the a-carbon. This elimination follows Zaitsev's rule whereby the more substituted alkene is generally formed. In E2 elimination, both the B-hydrogen and the leaving group must be oriented anti to each other. The same reaction…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY