
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
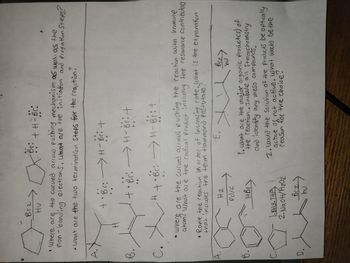
Transcribed Image Text:0
a
+H=BC:
Where are the curved arrow poshing mechanism as well as the
non-bonding electrons. What are the initiation and progation Steps?
•What are the two termination Steps for the reaction.
B.
с
A.
В.
C.
D.
Brz
HV
-
+ • Br: → H-Br: +
→
+ • Br: H-Br: +
H + • Br: → H-BC: +
•Where are the curved arrows pushing the reaction with bromine
atom? What are the radical product including the resonance contributors
• Rane the reaction in order of increasing rate. what is the explanation
that includes the term Hammond Poštutate?
H2
PJ/C
HBI
Br:
1.BH 3. THE
2. NaOHV/H₂O₂
Bcz
(2)
hu
x
1. What are the major organic productes) of
the reaction. Include all Stereochemistry
and identify any meso compounds.
E..
все
hu
2. Would the solution of the products be optically
active of not active. what would be the
Ceason for the choice?
Expert Solution
arrow_forward
Step 1
Given that, a reaction scheme is shown below
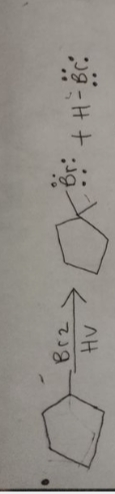
We have to draw the curved arrow reaction mechanism and have to draw two termination reaction.
Introduction: Radical Bromination reaction of alkane.
N.B.: According to the Bartleby Q&A guidelines, we have to answer only the first parts. So, dear student kindly post the remaining questions.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which reaction pathways shows the effect a catalyst (black) might have on an uncatalyzed reaction (gray)? O al O b) Od HAL O el mart Reaction pa Reaction path d) Both B and C are possible Ropatharrow_forwardEnergy 7. Consider the following energy diagram: (CH3)3C-OH (CH3)3C-ÕH₂ + HI + r a) Write the overall balanced chemical equation. Reaction coordinate (CH3)3C+ + H₂O + F b) How many steps are in the reaction mechanism? c) How many intermediates are in the reaction mechanism? d) Which step requires the most energy? (say step "X", where "X" is a number) e) CIRCLE ONE ANSWER. The overall reaction is an example of: substitution addition elimination (CH3)3C-I rearrangementarrow_forwardMany biochemical reactions are catalyzed by acids. A typical mechanism consistent with the experimental results (in which HA is the acid and X is the reactant) is Step 1: Fast, reversible HA = H+ + A- Step 2: Fast, reversible X + H+ = HX+ Step 3: Slow HX+ = productsWhich step controls the reaction rate?arrow_forward
- Predict the product of the following reaction mechanismsarrow_forwardRemaining Time: 1 hour, 16 minutes, 57 seconds. v Question Completion Status: A Moving to another question will save this response. Question 22 Arrange the following compounds in order of increasing reaction rate with HNOg04 2 1 O 3<1<2<4 O 3<4<1<2 O1<3<4<2 O1<2<3<4 A Moving to another question will save this responsearrow_forwardWhich of the following mechanisms is consistent with the energy diagram shown below: Energy Progress of the reaction A+B ---> C O 2A---> C (fast) B+C -> D (slow) A+B ---> C (slow) C---> D (fast A---> B (slow) B ---> C (fast) C---> D (fast)arrow_forward
- what is the mechanism of this reaction?arrow_forwardprovide full reaction mechanism and state where the equilibrium shiftsarrow_forward•OOH H H atom abstraction Consider the hydrogen atom abstraction step in an autooxidation mechanism, as shown above. Which of the following represents the intermediate that is formed in this step? OOH ? .00.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY