
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Follow the arrows to predict the intermediate and product of this reaction. Include all lone pairs. Use wedges and dashes to indicate stereochemistry. Ignore inorganic byproducts.
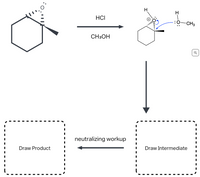
Transcribed Image Text:H
HCI
:0-CH3
CH3OH
neutralizing workup
Draw Intermediate
Draw Product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe (meaning what you would do and observe) simple (not including melting points, boiling points, cleaving reactions, or instrumental analyses) chemical tests(if any) that would distinguish between the compounds named below. [hint doing a table may be helpful] A. 1,3-pentadiene and 1-pentyne B. Ally bromide and 2,3- dimethyl-1,3-butadienearrow_forwardBalance the following chemical equation (if necessary): Ca(OH)2(s)+H3PO4(aq)4>H2O(I) +Ca3(PO4)2(aq)arrow_forwardExplain the reason that increasing and decreasing the volume of a gas will affect the reaction rate.arrow_forward
- 4. Show structures for the products formed in the following reactions. Assume monosubstitution. If more than one product is possible, show all of them, and indicate the major product (wherever relevant). If a reaction cannot occur, indicate "No Reaction". t-butylbenzene + 1-chloropropane, AlCl3 nitrohenzene + chloromethane, AlCl3 nitrobenzene + HNO3, H₂SO4, Aarrow_forwardDescribe (meaning what you would do and observe) simple (not including melting points, boiling points, cleaving reactions, or instrumental analyses) chemical tests (if any) that would distinguish between the compounds named below. [ Hint: using a table may be helpful ] A. 1,3-pentadiene & 1-pentyne B. allyl bromide & 2,3-dimethyl-1,3-butadienearrow_forwardDraw a structural formula for the major organic product of the reaction shown below. Br + CuLi • Consider E/Z stereochemistry of alkenes. • Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. • Do not include organocopper or inorganic ion by-products in your answer. P. opy aste ▼ [片arrow_forward
- Complete the following reactions. Only include the major products and any byproducts (H2O) but no minor products. Use either full structural diagrams or the combination method and don't use Skeletal/line diagrams.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediate and product of this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Use wedges and dashes to indicate stereochemistry. Ignore inorganic byproducts.arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Please don't provide handwriting solutionarrow_forwardFor the following reactions, fill in the boxes with the predominant product or products. You must indicate stereochemistry with wedges and dashes. If a racemic mixture is created, you must write "racemic" under the structures.arrow_forwardDraw the predominant product(s) of the following reactions including stereochemistry when it is appropriate. 2 HBr CH3-CEC-CH3 + • Consider E/Z stereochemistry of alkenes. • Do not show stereochemistry in other cases. If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. WP ***** ? ChemDoodleⓇ n [F < 56arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY