
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Identify and encircle the peptide bonds in this polypeptide (Ala-Leu-Glu-Ala-Asn-Asp-Arg-Ala)?
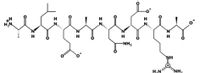
Transcribed Image Text:H
H
H
N,
H
H
H
-NH2
NH
H,N
NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw out the peptide DPGS as it would appear in a Type II β-turn and clearly label/identify all the Hydrogen bonds present between atoms (as well as other characteristic features of the β-turn). Be sure to draw out the entire peptide and label the N- and C-termini. Which residues in this peptide are characteristic of this secondary structure and what do they do?arrow_forwardPlease don't provide handwriting solutionarrow_forwardIn the molecule of oligomeric protein there are 19 lysine residues. 12 of them may be easily acetylated with anhydrides of dicarbon acids (it react with NH2-groups). The acetylation of extra two residues of lysine will dissociate the protein to the subunits. The rest 5 lysine residues may be modified only after denaturation of the protein. Suggest, how many lysine residues are: a) on a surface of protein globule; b) inside globule: c) in a site which is responsible for the contact within subunitsarrow_forward
- What is the 1-letter code for the amino acid glutamine? N E Q Garrow_forwardDraw the structure of Pro-Leu-Glu at physiological pH (7.4).arrow_forwardIn each of the following pairs of amino acids, identify which amino acid would be more soluble in water: (a) Ala, Leu; (b) Tyr, Phe; (c) Ser, Ala; (d) Trp, Hisarrow_forward
- LEFT:You have the following titration curve of an amino acid with a non-polar R-group with arrows on curve from (1) to (5). RIGHT: This amino acid has the following structures (a) to (e)) at different points of the titration curve. Match each numbered arrow on the curve (from (1) to (5)) with the appropriate amino acid structure (from (a) to (e)) 12 (5) H.NCHCOH + HNCHCO (a) 10 (4) CH3 CH Isoelectrin HŃCHCO + (b) ppint 43) HNCHCO CH3 CH3 (2) HaNCHCOH H. NCHCO (e) (d CH3 CH3 0.0 0.5 1.0 15 20 NaOH equivalents. H-NCHCO (e) CH3arrow_forwardWhat does this statement mean: "Upon folding of a protein many main chain amides replace their hydrogen bonds to water for those with other amide bonds" Is this true for alpha helix in proline racemase? and is there a pattern?arrow_forwardList some of the possible combinations of α-helices and βsheets in supersecondary structures.arrow_forward
- Given the polypeptide chain below: Ala-Arg-Val-His-Asp-Gln 1. What kind of polypeptide is it? 2. How many peptide bonds are there?arrow_forwardDraw a peptide from the given amino acids Phe-Cys-Ala-Arg-Ala-Ser-Tyr. Try to cleave this oligopeptide while it is travelling through your digestion tract. Can someone please explain the question for me and how to draw a peptide?arrow_forwardAmong these amino acid combinations listed above, only the combination of Lys and Glu have side chains with groups that have the greatest ability to stabilize the tertiary structure of a protein. Explain by drawing (a) why Lys and Glu side chain interaction stabilizes the tertiary structure of a protein (b) why the pairs of Glu and Asp & Arg and Pro cannot provide the stability to the protein structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON