
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Many reactions involve a change in hybridization of one or more atoms in the starting material. In following reaction, identify the atoms in the organic starting material that change hybridization and indicate the change.
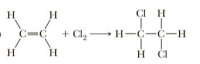
Transcribed Image Text:H
H
CI H
C=C
+ Cl, H-C-C-H
H
H
H ČI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- N CH3 K₂Cr₂O7 H* Iarrow_forwardWhat type of compound is this? H CH, C-N-CH₂arrow_forwardIntermolecular Forces and Solubility: (Like dissolve like) 1. Which of the following molecules are polar? Label them. 2. Which of the following molecules are nonpolar. Label them. 3. Which of the following compounds are ionic? Label them. 4. Which of the compounds below would dissolve best in cyclohexane and which ones will dissolve best in water? Put a W or C next to each indicated structure to indicate their solubility. Do not put W or C next to cyclohexane or water because they are solvents to dissolve other molecules H2 H2C° CH2 H. H2C. .CH2 water H2 cyclohexane K* i. H2 H2 H3C. K* CH3 H2 H2 H3C CH3 potassium sulfate hexane acetone K,SO4arrow_forward
- CH3 The structure of the amino acid L-alanine is shown. *H3N° Identify the functional groups that are common to all amino acids. O COO L-alanine CH; O H O NHarrow_forwardWhich functional group suffix would be used for the following organic compound? * CH, CH, H;C CH2 CH || alcohol (-ol) aldehyde (-al) ester (-oate) O ketone (-one) carboxylic acid (-oic acid)arrow_forwardHO C SH I CH 2 NH 2 -CH-CH 2 -CH 2 -C NH —CH —C Glutamic acid |NH —CH 2-C OH Glycine The molecule depicted above is a tripeptide. Here is another representation of it. The amino acid in the middle isarrow_forward
- Answer the questions in the table below about this molecule: CH3 O || HẠN—CH-C-NH-CH-C-NH-CH-C007 T | CH CH3 CH3 O || CH₂ | CH CH3 What kind of molecule is this? I CH₂ T C If you said the molecule is a peptide, write a description of it using 3-letter codes separated by dashes. O peptide O lipid O phospolipid 0 amino acid none of the abovearrow_forwardCH3CH,CH,CHCH,CH,CH3 CH H₂C CH3 Spell out the full name of the compound.arrow_forwardClassify each of the molecules in the table. molecule CH3CH₂CH=CH-CH₂-CH=CH (CH₂)6 O || C-OH CH3 CH₂-CH=CH-CH₂-CH=CH-(CH₂)4 —CH=CH—(CH₂)3 - CH3 - (CH₂)5 C-OH C-OH type of molecule (check all that apply) fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated w-3 w-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated w-3 w-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated w-3 w-6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning