
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For equilibrium constant of the given reaction why is hydrogen and methane included and diamond excluded?
Conditions of the reaction are: temperature=298K and pressure=1000atm
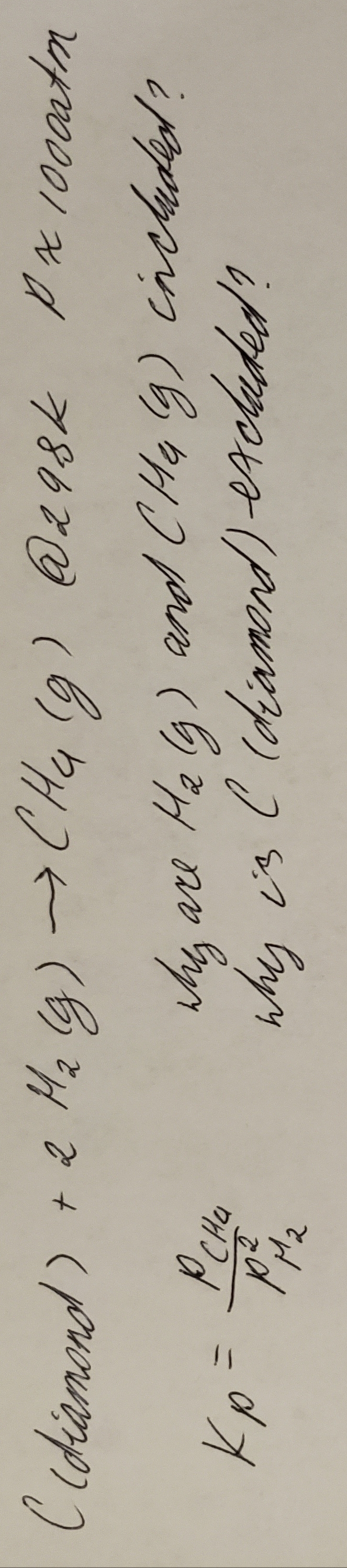
Transcribed Image Text:Gy's
es aclad
Cdamond) -e
(puuat
( 63H
aril i 78670
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation neededarrow_forwardThe dependence constant of the Of a reaction is ntcmparature given by the egoation_EaRT under what condition k the the smallest? as high I and Large Fa b.y hish I and smatt Eg low T and Lage Es and smallarrow_forwardTrue of false? There is only one value for the reaction quotient of a given reaction at a given tempaturearrow_forward
- Calculate the equilibrium constant for the reaction ammonia combining with oxygenforms nitrogen dioxide and water all the reactants and products are in gaseous state at425oC. If the initial number of moles in a 2.00L container for ammonia, oxygen , nitrogendioxide and water are 8mol, 12mol, 2 mol and 3mol respectively. The equilibriumconcentration of water vapour is 3.00mol/Larrow_forwardphysical chemarrow_forwardGive detailed solution..show work..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Give detailed Solution..show work..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardPlease don't provide handwritten solution .....arrow_forwardFor the equilibrium reaction. 2IBr (g) I2 (g) + Br2 (g) Kc=.0085. If .025 M of IBr is introduced to an empty flask and allowed to reach equilibrium, calculate the final concentrations of all components. Consider the decomposition reaction at 555 K 4POCl3 (g) P4 (g) + 2O2 (g) + 6Cl2 (g) If .450 atm of POCl3 is introduced to an otherwise empty flask and the reaction is allowed to reach equilibrium, the final total pressure is .850 atm. Find Kp and Kc.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY