
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
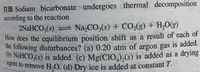
Transcribed Image Text:agent to remove H,0. (d) Dry ice is added at constant T.
17.55 Sodium bicarbonate undergoes thermal decomposition
according to the reaction
2NaHCO3(s) Na,CO,(s) + CO(g) + H,0(g)
How does the equilibrium position shift as a result of each of
the following disturbances? (a) 0.20 atm of argon gas is added.
) NaHCO,(s) is added. (c) Mg(CIO,),(s) is added as a drying
gent to remove H,O. (d) Dry ice is added at constant 1.
.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of NO decomposes according to the following equation: 2 NO(g) 1 N₂(g) + 1 O₂(g) An equilibrium mixture in a 8-L vessel at 8281 °C, contains 0.00187 g of NO, 0.00166 g of N₂, and 0.00136 g of O₂. (a) Calculate Kp for this reaction at this temperature. Kp = (b) What is the total pressure exerted by the equilibrium mixture of gases? Ptotal = atm.arrow_forward4) The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardGive detailed Solution with explanation needed...don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Explain thoroughly and answerarrow_forwardA sample of IBr decomposes according to the following equation: 2 IBr(g) 1 I2(g) + 1 Br2(g) An equilibrium mixture in a 1-L vessel at 1926 oC, contains 0.00946 g of IBr, 0.0122 g of I2, and 0.00475 g of Br2.(a) Calculate KP for this reaction at this temperature.KP = (b) What is the total pressure exerted by the equilibrium mixture of gases?Ptotal = atm.arrow_forwardChoose the equilibrium constant expressions for the following reactions. a) 2SO2(g) + O2(g) 2SO3(g) Keq = b) CS2(g) + 4H2(g) CH4(g) + 2H2S(g) Keq =arrow_forward
- At a given temperature, the equilibrium constant Kc for the reaction below is 2.37x103. 2S0, (g) +0,(g) – 2SO3 (g) What is the value of the equilibrium constant for each of the following reactions at that temperature?arrow_forward4. Toluene, C;H8(1), is an important organic solvent. It is made industrially from methylcyclohexane, C,H14(g): C,H14(g) + heat ? 2C;H8(1) + 3H2(g) State three different changes to an equilibrium mixture of these reacting gases that would shift the reaction toward greater production of toluene. State and explain each change.arrow_forwardA chemical engineer is studying the following reaction: HCH;CO,(aq)+CH;NH,(aq) → CH;CO,(aq)+CH,NH,(aq) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.73. The engineer charges ("fills") four reaction vessels with acetic acid and methylamine, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction compound concentration expected change in concentration vessel HCH;CO, 1.18 M f increase I decrease (no change) CH, NH, 0.97 M f increase OI decrease O (no change) A CH,CO, 0.39 M f increase O I decrease (no change) + CH,NH, 0.39 M f increase Ot decrease (no change) HCH,CO, 1.15 M f increase OI decrease O (no change) CH,NH, 0.88 M t increase I decrease (no change) В CH,CO, 0.86 M f increase OI decrease (no change) CH,NH,…arrow_forward
- Water gas is a 1:1 mixture of carbon monoxide and hydrogen gas and is called water gas because it is formed from steam and hot carbon in the following reaction: H2 O(g) + C(s) ⇌ H2(g) + CO(g). Methanol, a liquid fuel that could possibly replace gasoline, can be prepared from water gas and hydrogen at high temperature and pressure in the presence of a suitable catalyst.(a) Write the expression for the equilibrium constant (Kc) for the reversible reaction2H2(g) + CO(g) ⇌ CH3 OH(g) ΔH = −90.2 kJ(b) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more H2 is added?(c) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CO is removed?(d) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CH3OH is added?(e) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if the temperature of the system is increased?(f) What will happen to the concentrations of H2, CO,…arrow_forwardA chemical engineer is studying the following reaction: HCH;CO,(aq)+CH,NH,(aq) → CH;CO,(aq)+CH,NH,(2q) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.6. The engineer charges ("fills") four reaction vessels with acetic acid and methylamine, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction compound expected change in cor concentration concentration vessel HCH,CO, 0.96 M O T increase OI decrease O (no change) CH, NH, 0.88 M O f increase OI decrease O (no change) A CH,Co, 0.54 M O f increase OI decrease O (no change) CH,NH, 0.45 M O f increase OI decrease O (no change) HCH, CO, 0.88 M f increase OI decrease O (no change) CH,NH, 0.77 M O f increase OI decrease O (no change) B CH, Co, 1.09 M O increase OI…arrow_forward2) For the reaction, PCI:(g) = PCl:(g) + Ch(g) at 327°C, the equilibrium constant, Ke is 0.234. Suppose that 2.082 g PCIIS is placed in an evacuated 200 mL bulb, which is then heated to 327°C. (R = 0.082 L atm mol'K', PCIS = 208.2 g/mol) (a) What would be the pressure of PCIs if it did not dissociate? (b) What is the partial pressure of PCIS at equilibrium? (c) What is the total pressure in the bulb at equilibrium? (d) What is the fraction of PCIS at cquilibrium?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY