
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer choices for first blank:
a. 4
b. 5
c. 6
d. 7
Answer choices for second blank:
a. a
b. b
c. c
d. d
![Figure 3-7 shows the IR spectrum of a compound with
formula C₂H10O. This compound has
[Select]
of units of unsaturation. A
reasonable structure would be
[Select]](https://content.bartleby.com/qna-images/question/03317af0-4144-4b0a-a13c-c65183a22c1d/1eaceff4-a13a-4d7c-8bbf-a54515db610e/vs9v1m_thumbnail.png)
Transcribed Image Text:Figure 3-7 shows the IR spectrum of a compound with
formula C₂H10O. This compound has
[Select]
of units of unsaturation. A
reasonable structure would be
[Select]
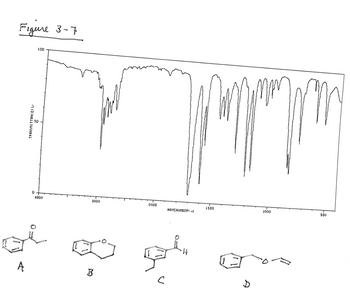
Transcribed Image Text:Figure 3-7
A
TRANSMITTANCEI
0
LOD
4000
3000
B
2000
mpr
www
S
HAVENUMBERI-11
H
1500
1000
500
Expert Solution
arrow_forward
Step 1: Units of unsaturation
Units of unsaturation is also known as degrees of unsaturation or index of hydrogen deficiency or sites of unsaturation.
Let's see calculation in next step.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The density of an object is 58.3 g/cm3cm3. Calculate the density in pounds per cubic inch (lbs/inch3inch3) A. 1.77 B. 4.29 C. 5.13 D. 2.11 E. 1.56arrow_forward#6arrow_forwardIf it appears that your 50 ml capacity burette is exactly at ten milliliters, how should this be recorded on the data sheet (i.e. significant figures to use)? a. 10 b. 10.0 c. 10.00 d. 10.000000arrow_forward
- An object will sink in a liquid if the density of the object is greater than that of the liquid. The mass of a sphere is 9.83 g. What is the minimum volume needed so that the sphere will sink in liquid mercury (density = 13.6 g/cm3). Select one or more: a. 134 b. none of the above c. 1.38 d. 7.48 e. 0.723arrow_forward7. A student calculates the density of a lead cylinder to be 10.3 g/cm3, but the actual density of lead is 11.3 g/cm3. What is the percent error of their measurement? a. 9.7% b. 8.8% c. 91.2% d. 90.3%arrow_forwardA liquid has the mass of 122 oz and density of 5.66 g/ mL. Calculate the volume in mL. A.) 616 mL. B.) 16.3 mL. C.) 125 mL. D.) 5.21 mL.arrow_forward
- Is this correct for this problem 7926 (4 sig figs) 6.4 926.381 Not sure how I would express it in scientific notationarrow_forwardMatch the correct prefix with the factor. A. 103 B. 109 C. 10-9 D. 10-2 V C E. 10-3 F. 102arrow_forward7. 12.0 mL of A weighs 10.8 g; and 16.0 mL of B weighs 14.4 g. A. A has a higher density than B. B. B has a higher density than A. C. A and B have equal densities. D. There is insufficient information to answer this question.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY