
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
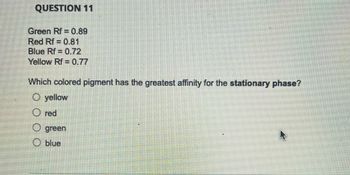
Transcribed Image Text:QUESTION 11
Green Rf = 0.89
Red Rf 0.81
Blue Rf = 0.72
Yellow Rf = 0.77
Which colored pigment has the greatest affinity for the stationary phase?
O yellow
Ⓒred
O green
O blue
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- The amino acid sequence (primary structure) and 3D shape (tertiary structure) of the rhodopsin has been determined and the various protein domains have also been identified with precision (see figures below). The red arrow indicates the amino acid lysin (K) that binds to the oxygen of the retinol (indicated by the blue arrow). The purple arrow indicates the retinal bound to the rhodopsin. In healthy individuals, retinal is abundant and can binds to the rhodopsin, making it functional (light-sensitive). H3C CH3 CH3 CH3 cytoplasmic side Retinal C-I extracellular side 0000 MOO OOO 400 DERY 000 PONE ALMO VOO LGF LLOD VUEL CH3 HOO AD SLV GO04 FAV OCK 000 LOO GOF YO DOAA TOTO ⓇAD MOWE OV00 OPL ALA LEGE DOOD VOOO COP DES 000 600 GCO OV FOOD Rhodopsin (primary structure) C-II E-II COOH-terminal C-III RIVE OOM DOWO LWCO 40 DOVO FOX ON DO 000 D04 boooooo 000 000 100,00 ****** E-III NH₂-terminal Rhodopsin + retinal (tertiary structure) 6. This lysine (K) is in a transmembrane domain of rhodopsin…arrow_forwardTWO PART QUESTION, PLEASE ANSWER BOTH, THANKS! The data seen are 4 various absorption wavelengths and it’s relative intensities. A. Using the data of relative intensity for 4 various absorption wavelengths, create a graph of the expected relative intensity for their respective emission wavelengths. Make sure to include all title and axis B. In only 2 sentences, explain your reasoning in your created graph abovearrow_forward○ Transition state analog Uncompetitive Mechanism-based Affinity-based O Non-specificarrow_forward
- A one-to-one protein (P)-ligand (L) complexation (P + L PL) has a dissociation equilibrium constant (Kd) value of 100 nM at 25°C, and the Kd remains the same at 37°C. 1) What is AS of binding at 25°C? Assume ACp of the binding is 0 over the temperature range. AS = 1.34E2 kJ/(mol*K) (note the unit!!) (sig. fig =3) 2) What is the concentration of the PL complex formed at equilibrium when you mix 0.20 uM (microM) of Protein and 1.0 uM of Ligand together at 37°C? PL at equilibrium = 8.1E-1 uM (note the unit!!) (sig. fig =2)arrow_forwardWould you say that the GadC anti porter exhibits the same pH dependence as the AdiC anti porter? If not which anti porter is less active at nonacid pHs?arrow_forwardWhen testing tonicity in potato strips, you soaked potato strips in different solutions. You were then able to determine the tonicity of the solutions based onarrow_forward
- Before and after for the image the color how is changedarrow_forwardWhat is the wavelength of a 6.76 x 1012 /s wave? Sorry im just really stuck on this question, Please dont leave any minor detail in the question, And also may u step by step this equation.arrow_forward#7 the highlighted structure is the ??arrow_forward
- 5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 a Dсм 23 Torr Symbols: в Dсм 50 Torr x DCM I00 Torr o, 0 Torr DCM; O, 23 Torr DCM A, 50 Torr DCM x, 100 Torr DCM. 80 60 40 (1 Torr = 1 mm Hg.) 20 The solutions were buffered to pH 7.4. 0.5 1.0 1.5 2.0 log p02 (a) (* Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the value of the dissociation equilibrium constant Ka for O2 binding is defined for 0 and 100 Torr of dichloromethane For the curves at 0 and 100 Torr of dichloromethane, draw their equivalent in the form of Kd Hb(O2)n Hb + nO2 % OXYGENATIONarrow_forwardThis lab examines the relationship between the absorbance of light by a solution at 595 nm and the concentration of the Coomassie Blue dye-BSA protein complex in the solution. State whether the following descriptions of the lab experiment are valid or not, and explain why you say Yes or No: a.The experiment would be significantly more accurate if absorbance readings were recorded for a range of wavelengths, not just for 595 nm.b.The experiment has limited accuracy because it does not account for the absorbance of light by the other components (components that are not the dye-protein complex, such as excess dye that is not bound to any protein) of the solution.c.The absorbance reading measures practically all the protein content in the solutionsarrow_forward5. n° of 2-chloro-2-methylpropane is 1.3850. If a 1g sample of 2-chloro-2-methylpropane was contaminated with 1 drop of ethanol, what do you think the effect would be on the refractive index and by approximately how much? (1 drop of ethanol weighs about 0.03 g).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education