
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Graph the data for the last time point at 75 minutes, and label each lines with the type of crystal
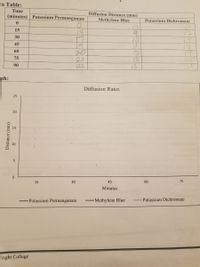
Transcribed Image Text:ta Table:
Time
Diffusion Distance (mm)
(minutes)
Potassium Permanganate
Methylene Blue
Potassium Dichromate
15
12
30
45
60
20
21
22
12
13
13
75
90
ph:
Diffusion Rates
25
20
15
30
45
60
75
Minutes
-Potassium Permanganate
Methylene Blue
Potassium Dichromate
/right College
Distance (mm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A substance has a melting point of -22°C and a boiling point of 75°C. What state will it be at the following temperatures? a. -75°C b. 125° C c. 25° C d. 0° C e. -15°C Blank # 1 Blank # 2 Blank # 3 Blank # 4 Blank # 5arrow_forwardNonearrow_forwardDecide which element probably has a boiling point most and least similar to the.boiling point of calcium. Comparing boiling point: gallium indium neon strontium most similar to calcium least similar to calciumarrow_forward
- 44. Match types of solid with each description. (Types of solids: molecular, metallic, ionic, covalent network) hard and brittle soft and brittle and nonconducting copper graphitearrow_forwardQuestion 12 of 20 Potassium chloride forms a solid made up of a highly organized set of positive and negative particles. Which term describes this solid? O A. An atom B. An ion C. A crystal D. A moleculearrow_forwardAnswer the attached photo.arrow_forward
- Give five practical applications each of crystalline and amorphous solids.arrow_forward= STATES OF MATTER Understanding consequences of important physical properties ... Liquid A is known to have a higher vapor pressure and higher surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment 35.0 mL of Liquid A are put in one sealed 5 L flask, and 35.0 mL of Liquid B are put in another sealed 5 L flask. The pressure in each flask is then slowly lowered with a vacuum pump. 35.0 mL of Liquid A are put in one sealed 5 L flask, and 35.0 mL of Liquid B are put in another sealed 5 L flask. The pressure in each flask is slowly increased by pumping in argon gas. O predicted outcome Eventually both liquids boil, A first and then B. Eventually both liquids boil, B first and then A. Neither liquid will boil It's impossible to predict whether eitherliquid boils without more information. Eventually both liquids boil, A first and then B. Eventually both liquids boil, B first and then A. Neither liquid will boil It's…arrow_forwardand compared to a pure substance. The melting point of an impure substance is usually A. higher B. over a broader range C. C lower D. over a narrow rangearrow_forward
- Chemistry Consider a liquid with a vapor pressure of 384 torr at 49.5°C and a vapor pressure of 1,245 torr at 129.3°C. What is the normal boiling point of this liquid in °C? (Hint: this is a two- step problem) Enter the normal boiling point of this liquid in °C to 1 decimal point.arrow_forwardClassify each solid as a covalent, ionic, metallic, or molecular solid. Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help frozen ethanol pure potassium potassium iodide red phosphorus Covalent lonic Metallic Moleculararrow_forwardMatch the solids given to the correct property. 10 Q F4 low melting point; soft high melting point; conducts when dissolved high melting point; malleable very high melting point; non conductor of electricity % F5 1. SIC c F6 2. Cl2 O7 (the letter after C is an L, not i) 3. Sn 4. MgCl₂ (the letter after C is an L, not i) MacBook Air & r AK F7 DII F8 C F9 Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY