
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
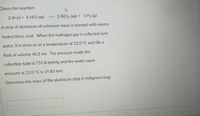
Transcribed Image Text:Given the reaction:
2 Al (s) + 6 HCI (aq)
--> 2 AICI3 (aq) + 3 H2 (g)
A strip of aluminum of unknown mass
reacted with excess
hydrochloric acid. When the hydrogen gas is collected over
water, it is done so at a temperature of 22.0 °C and fills a
flask of volume 40.3 mL. The pressure inside the
collection tube is 757.8 mmHg and the water vapor
pressure at 22.0 °C is 19.83 torr.
Determine the mass of the aluminum strip in milligrams (mg).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In order to produce solid iron, it first must bc processed from its orc, Fe2O1, in a blast furnace. During operation, CO gas (flue gas) is introduced into the furnace at 1650 °C and a pressure of 250 kPa. In this process flue gas reduces iron to its elemental form in the following reaction: 41. Fe;O30) COg Fe+ COz At one point in this process 256.0 L of CO was used to reduce iron ore. Assuming a complete reaction, how many grams of solid iron can be produced. Balanced chemical equation: Mass of Fe (in grams)arrow_forwardA chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder tha measures 52.0 cm wide and 62.4 cm high. The maximum safe pressure inside the vessel has been measured to be 7.00 MPa. For a certain reaction the vessel may contain up to 25.8 kg of sulfur hexafluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: C x10 Xarrow_forwardA sample of propane is placed in a closed vessel together with an amount of O2 that is 3.05 times the amount needed to completely oxidize the propane to CO2 and H2O at constant temperature. Calculate the mole fraction of each component in the resulting mixture after oxidation assuming the H2O is a gas.arrow_forward
- A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 26.0 cm wide and 31.2 cm high. The maximum safe pressure inside the vessel has been measured to be 2.40 MPa. For a certain reaction the vessel may contain up to 0.237 kg of dinitrogen monoxide gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. temperature: || °C Contacts G.. APR 24 80 888 F2 DII F3 F4 F7 F8 F9 F10arrow_forwardA tank was filled with 18 g of oxygen (O2), 75 g of nitrogen (N2) and 4 g of carbon dioxide(CO2). At 25°C the pressure of the tank was 8.5 atm. If the partial pressure of CO2 in the tank is x atm, what is the value of x?arrow_forwardA student experimentally determines the gas law constant, R, by reacting a small piece of magnesium with excess hydrochloric acid and then collecting the hydrogen gas over water in a eudiometer. Based L-atm on experimentally collected data, the student calculates R to equal 0.0832 mol·K L-atm Ideal gas law constant from literature: 0.08206 mol·K (a) Determine the percent error for the student's R-value. Percent error = % (b) For the statements below, identify the possible source(s) of error for this student's trial. The student notices a large air bubble in the eudiometer after collecting the hydrogen gas, but does not dislodge it. The student does not clean the zinc metal with sand paper. The student does not equilibrate the water levels within the eudiometer and the beaker at the end of the reaction. The water level in the eudiometer is 1-inch above the water level in the beaker. The student uses the barometric pressure for the lab to calculate R.arrow_forward
- When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable limestone. Suppose some calcium carbonate is sealed into a limekiln of volume 550. L and heated to 520.0 °C. When the amount of CaCO3 has stopped changing, it is found that 8.46 kg have disappeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO3 and CaO at 520.0 °C. Round your answer to 2 significant digits. P Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value. 0 Xarrow_forward5.(a) Assume for the moment that air consist of 80.00% nitrogen and 20.00% oxygen byvolume. Use these figures and typical values for temperature and pressure (1.000atm, 25.0 ºC) to calculate the density of air. (b) This calculation affords a good approximation of the density of air at sea level.However, your lab is at an altitude approximately 777 meters (2550 feet). Knowingthis, will the density of air in you lab be higher or lower than the value you justcalculated? Why? (c) Besides altitude, what else affects the atmospheric pressure? Where could you findthe actual atmospheric pressure at the time of the experiment?arrow_forwardWhen limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete the lime absorbs CO₂ from the air and turns back into hard, durable 2 limestone. Suppose some calcium carbonate is sealed into a limekiln of volume 400. L and heated to 740.0 °C. When the amount of CaCO3 has stopped changing, it is found that 3.37 kg have disappeared. P 00. Calculate the pressure equilibrium constant K this experiment suggests for the equilibrium between CaCO3 and CaO at 740.0 °C. Round your answer to 2 significant digits. Ar Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value. р K₁ = 0 x10 р x Ś ? Explanation Check 0 81 K © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY