
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
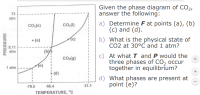
Transcribed Image Text:Given the phase diagram of CO2,
answer the following:
a) Determine F at points (a), (b)
(c) and (d).
b) What is the physical state of
CO2 at 30°C and 1 atm?
c) At what I and P would the
three phases of CO2 occur
together in equilibrium?
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider the following chemical equilibrium: HCOOH(aq) + H_2O(aq) \rightleftharpoons HCOO^-(aq) + H_3O^+(aq). Which of the following graphs represents the perturbation on the system and the change of pH when water is evaporated from the container?arrow_forwardConsider the following equilibrium system: N2 (g) + 3H2 (g) Ź 2NH3 (g) + 92.94 kJ For each, state what happens to the number of moles of nitrogen gas, N2, and why that occurs. (the shift) a. increasing the temperature f. removing some ammonia b. decreasing the temperature g. adding some ammonia c. decreasing the pressure h. removing some nitrogen с. 6.arrow_forwardTwo liquids A and B mix to form an ideal solution. Consider A to be the solvent (majority component) and B to be the solute (minority component). What is the relationship between the Gibbs energy and chemical potential for a pure phase? How does the Gibbs energy of A(solution) COmpare to Gibbs energy of Apure liquid)? How does the Gibbs energy of B(solution) compare to Gibbs energy of B(pure liquid)? d. When B is added to pure A, what happens to the Gibbs energy of A? а. b. C. e. Why does the Gibbs energy of the solvent of an ideal solution (in this case, A) decrease upon the addition of a solute (in this case, B)? Provide mathematical and conceptual explanations.arrow_forward
- Question 1. Derive an expression for the vapor density at the boiling point as a function of temperature. Assume that the ideal gas law is valid. The vapor density of water at 25°C is 23.4 g/m³. Calculate the vapor pressure and vapor density at 50°C.arrow_forwardSolve the following questions related to fugacity of a liquid. Assume that liquid is incompressible and thus the = 1 molar volume of the liquid is constant. (You may assume that Psat is low enough that sat for all parts of this problem. You will also need to look up or calculate the molar volume of water.) You are given a container partially filled with water at 25°C. You then proceed to pump all of the air out of the container, leaving a pool of water at the bottom, and a vacuum (P=0) at the top (see first panel in figure below). You then turn off the pump and seal the container. You wait a short while and the water in the bottom starts to evaporate (see second panel) until it achieves phase equilibrium (see third panel). Assuming no change in temperature and no significant change in volume of the water, what is the pressure in the box? (Hint: you must balance fugacity between the liquid and vapor phases. You may also assume the vapor phase is an ideal gas, and the liquid is an ideal…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The