
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
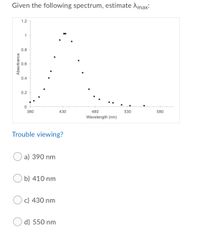
Transcribed Image Text:Given the following spectrum, estimate Amax:
1.2
1
0.8
0.6
0.4
0.2
380
430
480
530
580
Wavelength (nm)
Trouble viewing?
a) 390 nm
b) 410 nm
c) 430 nm
d) 550 nm
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the meaning of the following terms and phrases: 1.1 Precision of a set of results. 1.2 Noise around an analytical signal. 1.3 A shelf-life of an aqueous trace metal standardarrow_forwardThe ¹H NMR spectrum of a compound with the formula C12H2406 is pictured below. Determine the structure of the molecule using as much information as possible from the spectrum. Record your calculations and reasoning in the box given below the spectrum 10 9 -00 8 7 T 6 T 5 4 T 3 T 2 1 0arrow_forwardA student creates a sports drink by dissolving 5.0 g of powder in 250 mL of water. The green colour of the drink is due to the presence Green Dye X, which has a lambdamax of 630 nm. A calibration curve (absorbance versus concentration) at lambdamax is created for varying concentrations (mM) of Green Dye X which has the equation y = 26.4x. If 1.0 mL of the sports drink is diluted to 10. mL with water, then the absorbance is measured to be 0.450, what is the mass percent of Green Dye X in the drink powder if the molecular weight is 418.4 g mol-1?arrow_forward
- A student creates a sports drink by dissolving 5.0 g of powder in 250 mL of water. The green colour of the drink is due to the presence Green Dye X, which has a lambdamax of 630 nm. A calibration curve (absorbance versus concentration) at lambdamax is created for varying concentrations (mM) of Green Dye X which has the equation y = 26.8x. If 1.0 mL of the sports drink is diluted to 10. mL with water, then the absorbance is measured to be 0.340, what is the mass percent of Green Dye X in the drink powder if the molecular weight is 418.4 g mol-1?arrow_forwardWhat is the wavelength in nm of 7.47*10-19arrow_forwardLogically deduce the structure of compound 1B whose Spectra are given: Molecular formula C4H8Oarrow_forward
- Topic: Transmittance and absorbance, lambert-beer law Ketorolac Tromethamine (C15H13NO3 • C4H11NO3) has an absorbance of 0.615 at 318.6 nm and 1 cm optical path in 3.5 µg / mL concentration solutions. A 2.9252 g sample from a tablet is diluted with water to 250 mL. They take 25 mL add 3.0 mL of 0.1 N H2SO4 and dilute to 500 mL. A 10 mL aliquot is taken and diluted to 200 mL. Of the latter solution, the absorbance at 318.6 nm is measured, resulting in 0.523. Calculate the milligrams of Ketorolac Tromethamine for each compressed. Fact: Average weight of 6 tablets equals 1924.5 mgarrow_forward#5) Using the following spectra and other information provided, identify the compound. Assign pertinent peaks in the spectra. Label the peaks on the spectrum and place the structure of the compound in the box on the right side of the spectrum - Identify correct compound - label proton spectrum - label carbon spectrumarrow_forwardA compound has a strong absorption at 8.37x1015 Hz. What is the Amax( in Å (angstroms)) for this compound? (Write answer in decimal form to one decimal place, for example, if answer is 75.667 then write as 75.7)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY