
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Does the spectrum math the structure below?
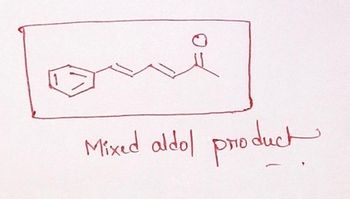
Transcribed Image Text:Mixed aldol product
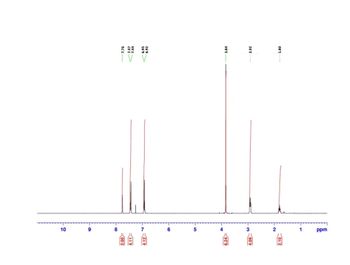
Transcribed Image Text:2.00
4.11
4.12
6.24
4.09
2.10
10
9
8
6
5
4
3
2
1
ppm
7.76
7.47
7.44
6.95
6.92
3.84
2.92
1.80
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi, I'm in need of some help with this.arrow_forwardNEED HELP NOT GRADED PRACTICEarrow_forwardProblem # 2 Determine the structure of the compound with the spectra provided and molecular formula C5H100. I 200 180 160 T 10 9 8 2000 140 120 1100 7 6 5 100 80 4 60 1000 3H singlet 6H doublet 37 1H septet 3 2 за 40 20 0 1 500 0arrow_forward
- 1. Draw two constitutional isomers of the formula C6H11B1. Explain how you would differentiate those molecules using either spectroscopy or spectrometry. You only need to differentiate them using one example.arrow_forwardRank the labeled protons in order of increasing chemical shift.arrow_forward3.What peaks would expect to find for you Vs, cie'thy) amine thy) in the InfraRed' spectrum? +-butyl 'amine us. n-butyl aminearrow_forward
- Look at the three infrared spectra in Figures C to E and answer the following questions (a) Are any of the spectra that of an alcohol? If so, which? What absorption pattern(s) at what wavelength(s) identifies an alcohol? (b) Are any of the spectra that of a compound containing a benzene ring? If so, which? What three absorption patterns at what wavelengths show that a compound has a benzene ring? (c) Are any of the spectra that of a compound containing only carbons and hydrogens? If so, which? Benzene rings contain only carbons and hydrogens. Might the spectrum or spectra you chose for your answer above indicate a benzene ring? (Tell what absorption patterns are present or not present that would support your answer.)arrow_forwardProblem # 1 Determine the structure of the compound with the spectra provided and molecular formula C4H8O. 3000 10 200 180 160 140 9 2000 8 7 120 100 80 6 4500 5 4 60 2H 3 mm 2 1000 40 3H 20 3H 1 0 0arrow_forwarda) Propose structures for compounds of the following molecular formulas, using the spectra given below.Show your workarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY