
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
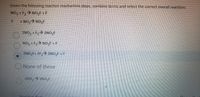
Transcribed Image Text:Given the following reaction mechanism steps, combine terms and select the correct overall reaction:
NO, + F, > NO,F +F
fON ON +
ZNO, +F,> 2NO,F
NO, 1E,> NO,F +F
2NO,F+ 2F,> 2NO,F+F
ONone of these
Dresn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction is run at two temperatures where temperature 1 is lower than temperature 2. Which relationship is correct for either rate constant (k1 or k2) or activation energy (Ear1 and Ea.2)? O k1 k2 O Ea,1 Ea,2arrow_forwardThe following initial rate data are for the reaction of nitrogen monoxide with ozone at 25 °C: NO + 03 NO, + 02 Experiment NO], M [03lo, M Initial Rate, Ms! 9.31x10-3 1.86x10-2 9.31x103 1 0.113 9.56x10-2 2 0.113 0.191 0.226 0.191 4 0.226 1.86x10-2 0.382 Complete the rate law for this reaction in the box below. Use the form k[A]"[B]" , where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n Rate = From these data, the rate constant isarrow_forwardThe following reaction occurs by the Mechanism below: B + B ---> X (slow) X + A ---> Y (fast) Y + B ---> C (fast) what is the rate law? what is the overall balanced equation? identify an intermediate in the equationarrow_forward
- Analyze the following reaction mechanism: H2O + O → CIÓ + CF2CI CI + 202 4. Cl + CF2CI –→ CF2CI2 1. H2O2 2. O + CF2CI2 3. CIO + Oз -> Identify the reaction intermediates in this reaction. A) O, CIO, CI B) CI, CF;CI, CF2CI2 C) O, CIO, O, H20 D) O, CIO, CI, CF2CI E) O, CIO, CI, CF;CI2arrow_forwardAnalyze the following mechanism: What are the intermediates and catalysts if there are any? What is the overall reaction?arrow_forwardThe following data were obtained for the reaction A + B + C⟶⟶ products: Expt # [A]0 [B]0 [C]0 Initial rate, (mol L-1 s-1) 1 1.25 x 10-3 M 1.25 x 10-3 M 1.25 x 10-3 M 0.0087 2 2.50 x 10-3 M 1.25 x 10-3 M 1.25 x 10-3 M 0.0174 3 1.25 x 10-3 M 3.02 x 10-3 M 1.25 x 10-3 M 0.0508 4 1.25 x 10-3 M 3.02 x 10-3 M 3.75 x 10-3 M 0.457 5 3.01 x 10-3 M 1.00 x 10-3 M 1.15 x 10-3 M ? From this data calculate the overall order of reaction. Group of answer choices 1 2 3 5 6arrow_forward
- Give typed full explanation not writtenarrow_forwardWhich one of the following is not a valid expression for the rate of the reaction below? 4NH3 + 702 + 4NO2 + 6H20 O 1 A[02] At O 1 A[NO2] 4 At O 1 AH20] At Ο 1ΔΝΗ 3] 4 At O all of these are valid expressions of the reaction rate.arrow_forwardAnalyze the following reaction mechanism: 1. H₂O2 → H₂O + O 2. O + CF₂Cl2 → CIO + CF₂CI 3. CIO + O3 → CI + 20₂2 4. CI+ CF₂CI → CF₂Cl2 Identify the reaction intermediates in this reaction. A) O, CIO, CI B) CI, CF₂CI, CF₂Cl2 C) O, CIO, O, H₂O D) O, CIO, CI, CF₂CI E) O, CIO, CI, CF₂Cl2 ULLarrow_forward
- 2 H₂ NO H₂O 2 H₂0 The reactants in the overall reaction are (include their correct coefficients): 2 NO 2 N₂0 N₂ N₂0 H₂ 2 N₂0₂ 2 N₂ Consider the following proposed reaction mechanism: N₂02 Step 1: NO + NO N202 Step 2: N202 + H2 - N2O + H20 Step 3: N2O + H2-N2 + H2O The second step is the slow (rate-determining) step. Answer the following questions: What is the correct rate law for the overall reaction? O rate = K[NO]²[H₂1² rate = k[NO]²[H₂] O rate= K[N₂0₂][H₂] rate = K[N₂0₂]²[H₂] The products in the overall reaction are (include their correct coefficients): N₂ NO 2 H₂O H₂ U U U U 2 H₂ 2 N₂0 N₂02 2 NO N₂0 H₂O 2 N₂02 2 N₂ The intermediates in the mechanism are: N₂0 H₂O NO N₂ N₂0₂ H₂arrow_forwardThe reaction: A + 3B-> D + F was studied and the following mechanism was determined A + B -> C (fast) C + B-> D + E (slow) E + B -> F (very fast) The step with largest activation energy is None of the steps has an activation energy. the first step the third step All steps have the same activation energy. the second steparrow_forward5. The reaction A →B+C is known to be first order in A. Below are data showing the concentration of A as a function of reaction time. Time, min [A], M 5.00 x 10-2 4.30 x 10-2 5 10 3.70 x 10-2 2.74 x 10-2 1.51 x 10-2 20 40 1.12 x 10-2 8.26 x 10-3 50 60 (a) What is the average rate of reaction between 5 and 20 min? Report the units as well as the numbers. (b) Plot the data above, showing the concentration of A as a function of the time. From your graph, determine the instantaneous rate of reaction at 40 min. Report the units as well as the numbers. (c) Plot In [A] versus time. Estimate the rate constant for this first-order reaction from your graph. Report the units as well as the numbers.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY