
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
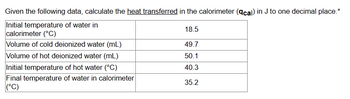
Transcribed Image Text:Given the following data, calculate the heat transferred in the calorimeter (\(q_{\text{cal}}\)) in J to one decimal place.
| Initial temperature of water in calorimeter (°C) | 18.5 |
|---|---|
| Volume of cold deionized water (mL) | 49.7 |
| Volume of hot deionized water (mL) | 50.1 |
| Initial temperature of hot water (°C) | 40.3 |
| Final temperature of water in calorimeter (°C) | 35.2 |
To calculate the heat transferred, use the known volume and temperature changes in the water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- #18arrow_forwardA student is asked to identify an unknown piece of metal. The metal has a mass of 12.34 g. It is placed in a boiling water bath and brought up to 99.98 oC. A coffee-cup calorimeter is set up with 103.25 mL of water (density = 1.00 g/mL and specific heat = 4.184 J/g.oC) at a room temperature of 21.30 oC. The metal is removed from the boiling water and placed in the calorimeter. A final temperature is recorded as 22.32 oC. Find the specific heatcapacity of the unknown metal. (Assume there is no heat loss)arrow_forwardPlease don't provide handwriting solutionarrow_forward
- In an experiment, 26.5 g of metal was heated to 98.0°C and then quickly transferred to 150.0 g of water in a calorimeter. The initial temperature of the water was 22.5°C, and the final temperature after the addition of the metal was 32.5°C. Assume the calorimeter behaves ideally and does not absorb or release heat.arrow_forwardspecific heat of gold is 0.129J/g C. what is molar heat capacity of gold?arrow_forwardAn 80.0g sample of metal, initially at 96 degrees C, is placed into 150g of water initially at 26 degrees C in a calorimeter. The final temperature of the water is 28.1 degrees C. What is the specific heat of the metal?arrow_forward
- A hot 122.8 g lump of an unknown substance initially at 154.8 °C is placed in 35.0 mL of water initially at 25.0 °C and the system is allowed to reach thermal equilibrium. The final temperature of the system is 56.9 °C. Using this information and the specific heat values for several metals in the table, identify the unknown substance. Assume no heat is lost to the surroundings. graphite zinc aluminum titanium tungsten rhodium Substance Specific heat (J/(g-°C)) aluminum 0.897 graphite 0.709 rhodium 0.243 titanium 0.523 0.132 0.388 4.184 tungsten zinc waterarrow_forwardThe temperature of a sample of water increases from 20C to 46.6C as it absorbs 5650J of heat. What is the mass of the sample?arrow_forwardWhen a 6.50 g sample of solid sodium hydroxide dissolves in 100.0 g of water in a coffee-cup calorimeter, the temperature of the water rises from 21.6 to 37.8oC. Was the chemical reaction (dissolving the solid) endothermic or exothermic? How do you know? Write a balanced chemical equation for this process. Determine how many joules of heat (q) were involved in changing the temperature of the water. If the heat that changed the temperature of the water was a result of the chemical reaction, determine the ΔH of the chemical reaction in kJ/mol of sodium hydroxide.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY