
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Image below
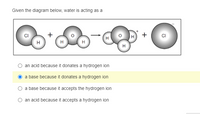
Transcribed Image Text:Given the diagram below, water is acting as a
+
+
H
H
H
H
O an acid because it donates a hydrogen ion
a base because it donates a hydrogen ion
O a base because it accepts the hydrogen ion
an acid because it accepts a hydrogen ion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help!!! Need correct solution with explanation.arrow_forwardMost alkenes absorb at a shorter wavelength than alkadienes. Explain.arrow_forwardPredict whether the MO diagram for S, would show s-p mixing or not. Solution No, any atom with four or more p electrons has paired electrons in the p orbitals so it does not mix, just like O.. Please answer correct fast pleasearrow_forward
- Part A Mark with the green check to indicate that the given direction of electron flow in the following set of molecules using curved arrows notation is correct and with the red X label to indicate when incorrect. Drag the appropriate labels to their respective targets. ► View Available Hint(s) X X X H₂C X CH₂ H₂C Reset Help You labeled 2 of 7 targets incorrectly. Electrons can only be shown to migrate if there is an atomic p orbital to move them to. Moving a lone pair electrons to form a new π bond can only occur if the atom acceptingarrow_forwardi need help with the last two structures on the left and right pleasearrow_forwardSs important values if needed for this question. In which of the following pure compounds would intermolecular hydrogen bonding be expected? (Select all that apply.) CH3CH2CH2CH2CH2COCH3 CH3CH2CH2CH2CH2COH H. CH2CH3 OCH3CH2CH2OH ONone of the Abovearrow_forward
- Could we cut just one bond in the "starting" molecule shown in the drawing area below to create this "target" molecule? The target molecule. If so, highlight the bond to be cut. If not, check the box under the drawing area that says Not possible. Note: it's OK if cutting the bond creates more than one molecule, as long as one of them is the target molecule. Not possible. X H H-C-H H-O-H H 3 Note for advanced students: what we mean by "cutting" the bond here is breaking the bond and attaching H atoms to each dangling end, like this: H H-C-0-H Harrow_forwarda) Draw one isomer of C6H14. b) Draw one isomer of C6H12- c) Draw one isomer of C6H140 that exhibits hydrogen bonding. d) Draw one isomer of C6H140 that is not capable of hydrogen bonding. BONUS: Show all locations of possible hydrogen bonding for the C6H140 isomer that you drew above in part c.arrow_forwardcan you please please answer everything in the image attach please write clearly and neatly and not messy so its easy to read and understand please answer fastarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY