
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
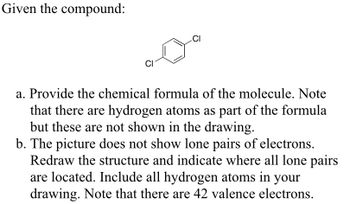
Transcribed Image Text:Given the compound:
CI
CI
a. Provide the chemical formula of the molecule. Note
that there are hydrogen atoms as part of the formula
but these are not shown in the drawing.
b. The picture does not show lone pairs of electrons.
Redraw the structure and indicate where all lone pairs
are located. Include all hydrogen atoms in your
drawing. Note that there are 42 valence electrons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis Dot structure for PBr3 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in PBr3? Express your answer as a whole number. b. How many single bonds are in PBr3? Express your answer as a whole number. c. How many double bonds are in PBr3? Express your answer as a whole number. d. How many triple bonds are in PBr3? Express your answer as a whole number.arrow_forwardNa F. Quick Comprehension Choices (highlight the correct choice) 1. Na (gained or lost an electron. 2. The Na atom now has an overall (negative or positive) charge. 3. F (gained or lost) an electron. 4. The F atom now has an overall (negative or positive) charge. 5. The atomns have (the same or opposite) charges and they (repel or attract) each other because of this force. 6. The compound NaF (does or does not) have an overall charge because the charges (cancel or add together).arrow_forwardQuestion 1 1 pt Convert the following molecular model into a skeletal structure. ball & stick + labels - • You do not have to explicitly draw H atoms. opy aste Carrow_forward
- Consider the molecule epinephrinea. Draw the structural formula. Show all atoms, bonds and lone pairsarrow_forwardCan you please name the molecule?arrow_forward1. What are functional groups? What are some common functional groups and how do functional groups affect the properties of an organic molecule? 2. What is the functional group on each of the molecules above?arrow_forward
- A fictional compound contains two elements. Here's what you know: Nyeon (X): a main group metal, has 3 valence electrons Tysonium (Y): a nonmetal, has 6 valence electrons A. Is this an ionic compound, a covalent compound, or an acid? B. What would the chemical formula of this compound be?arrow_forwardPlease answer all of the questions. Questions are in the image.arrow_forward4. What ionic compound name matches the formula represented by the picture? The small circles are cations, and the large circles are anions. a. Lithium bromide b. Sodium oxide c. Aluminum sulfate d. Calcium nitride e. Magnesium chloridearrow_forward
- the first image has instructions. please answer.arrow_forward1.Draw models for the molecule that will form between chlorine and hydrogen atoms. Electron Dot Diagram Structural Formula 2. Draw models for the molecule that will form between sulfur atoms. Electron Dot Diagram Structural Formulaarrow_forward3. Which of the following statements is true about the molecule shown below? 0- 0- O A. The bonds are polar and the molecule is polar. O B. The bonds are polar and the molecule is nonpolar. OC. The bonds are nonpolar and the molecule is polar. O D. The bonds are nonpolar and the molecule is nonpolar. 4. The image below shows a mixture of polar and nonpolar molecules. What type of intermolarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY