
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
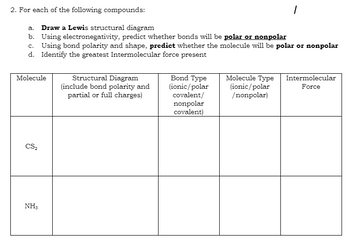
Transcribed Image Text:2. For each of the following compounds:
a. Draw a Lewis structural diagram
b. Using electronegativity, predict whether bonds will be polar or nonpolar
c. Using bond polarity and shape, predict whether the molecule will be polar or nonpolar
Identify the greatest Intermolecular force present
d.
Molecule
CS₂
NH,
Structural Diagram
(include bond polarity and
partial or full charges)
Bond Type
(ionic/polar
covalent/
nonpolar
covalent)
Molecule Type
(ionic/polar
/nonpolar)
Intermolecular
Force
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer fastarrow_forwardWhat is the relationship between the following compounds? A. constitutional isomers B. different representations of the same molecule C. different molecules D. isotopes E. resonance structuresarrow_forward1. Draw the best Lewis dot structure for the anion CCI in the correct molecular geometry [Include formal charges and lone pair electrons, and use dashed and solid wedge bonds if necessary 2. How many electron groups are present around the central atom and what is the electron group geometry? 3. What is the molecular geometry and ideal bond angles? 4. Is the molecule polar or nonpolar? If it is polar, draw a dipole moment arrow next to your structure to indicate the directionality of the dipole moment. Answers Edit View Insert Format Tools Table 12ptv Paragaph BIVA 2 Owordh AG3454jpg IMG 3450jpgarrow_forward
- The following five questions relate to this structure. All bonding pairs have been drawn, but lone pairs have been left out. Which atom(s) is/are expected to carry a partial negative charge in this structure? a. ND and OB b. OB only c. CE only d. CC and CE e. CC only f. ND only How many lone pairs are on Atom D? What is the steric number of atom C? What is the molecular geometry around atom A? a. See-saw b. Trigonal planar c. Tetrahedral d. Square planar Rank the given bond angles from largest to smallest. H—CF—CE OB—CC—CA H—CA—Harrow_forward2. This question uses sulfur trioxide and the sulfite ion and sulfur trioxide a. Draw both Lewis structures. b. Give the shape and geometry of each structure.arrow_forwardSteps for Lewis Structures: 1. Determine the total number of valence electrons. Add electrons for negative charges, subtract electrons for positive charges. 2. Place least electronegative atom (except H) as central atom in structure. 3. Connect atoms by singles bonds. Each single bond = 2 electrons. 4. “Sprinkle” remaining electrons around outside atoms first to complete octets. Don’t use more electrons than the total found in step 1. Then complete the central atom’s octet last if you have enough electrons. 5. Make double or triple bonds as needed to complete octets. 6. Place brackets and charge for ions. For the central atom in each formula, draw the Lewis Structure with all valence electrons shown. 1. PH3 2. H2O 3. CO2 4. CHCl3 5. O2 6. N2 7. CF4 8. C3H8 9. CH3COOH 10. N2O 11. OCN-arrow_forward
- Which of the following best describes electronegativity trends in the periodic table?I. Electronegativity inreases from right to left across a period.II. Electronegativity inreases from left to right across a period.III. Electronegativity inreases from bottom to top within a group.IV. Electronegativity inreases from top to bottom within a group. Select one: a. I and III b. I and IV c. II and III d. II and IV e. None of these.arrow_forwardSHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1. Is it possible for a molecule to be nonpolar even though it contains polar bonds? Explain your answer and give an example.arrow_forwardquestion 7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY