
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Given that the radius of a metal is 211.79 pm, determine the edge length for a body centered cubic composed of this metal.
Expert Solution
arrow_forward
Step 1
Crystal lattice is defined as the three dimensional arrangement if atoms or ions in a crystal. Various types of crystal lattices exist such as simple cubic, body centered, face centered, edge centered etc.
arrow_forward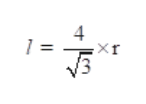

Step 2
A body centred crystal (bcc) lattice of metal is that which contains one atom at body center and eight atoms at the corners of the cube.
The relation between radius of atom (r) and edge length (l) of the crystal is given as:
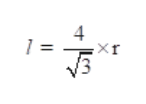
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the equation for calculating the face diagonal of a simple cubic unit cell. face diagonal =arrow_forwardBriefly compare the packing efficiency of the unit cells for a simple cubic unit, a body-centered cubic unit, and a face-centered cubic unit?arrow_forwardIt is not possible to pack spheres together without leaving some void spaces between the spheres. Packing efficiency is thefraction of space in a crystal that is actually occupied by atoms. Determine the packing efficiency of a face-centered cubic metal.arrow_forward
- 1. An ionic compound composed of chromium ions and oxide ions crystallizes into a face-centered cubic arrangement of chromium ions with oxide ions occupying all of the tetrahedral holes within the unit cell. What is the ionic formula for this compound? What are the oxidation states of the chromium and oxide ions? 2. Examine the ionic unit cell structure shown at right. The red spheres represent sulfide ions and the purple spheres represent nickel ions. Which ions define the unit cell? What kinds of "holes" are filled by the counter ions? What is the ionic formula for this compound?arrow_forwardWhat is the coordination number of an aluminum atom in the face-centered cubic structure of aluminum?arrow_forwardIf a metallic element crystallizes in a face-centered cubic unit cell with an edge length of 344.68pm, what is the radius in pm of an atom of this element in that structure? (enter your answer as the magnitude of the radius in pm, do not include the unit in the text of your answer) 121.86 121.9 60.93 1.4925*10^12 60.931 1.492*10^12arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY