
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Give detailed Solution with explanation needed..give answer all sub parts if you not then don't give answer..
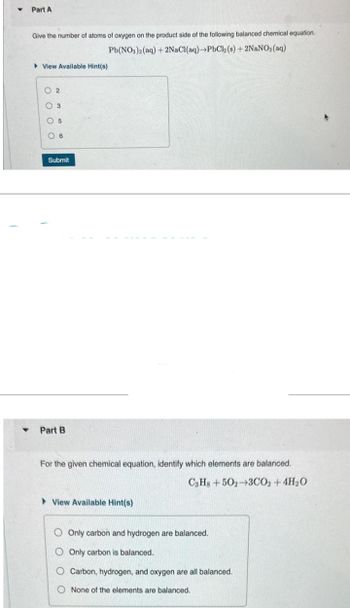
Transcribed Image Text:Part A
Give the number of atoms of oxygen on the product side of the following balanced chemical equation.
PbCl2 (s) + 2NaNO3(aq)
Pb(NO3)2(aq) + 2NaCl(aq)
View Available Hint(s)
02
3
05
6
Submit
Part B
For the given chemical equation, identify which elements are balanced.
C3H8+502-3CO2 + 4H₂O
View Available Hint(s)
Only carbon and hydrogen are balanced.
Only carbon is balanced.
O Carbon, hydrogen, and oxygen are all balanced.
None of the elements are balanced.

Transcribed Image Text:Part C
When the following chemical equation is balanced with all whole numbers, give the coefficients:
Al(s) + H₂SO4 (aq)→_Al2(SO4)3(aq) + H₂(g)
View Available Hint(s)
3,2,1,2
O2,3,2,3
2,2,2, 2
O2,3,1,3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give Detailed explanation Solution of all..choose with correct option...(don't give Handwritten answerarrow_forwardPeardeck Exarcase use #5, Given whats below find all [],'s and calculote K [], O.100 Charge 0,010 Students, draw anywhere on this slide! Pear Deck Interactive Slide Do not remove this bar Slide 1/1arrow_forwardGive clear detailed Solution with explanation needed..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Give detailed Solution with explanation needed with structures..don't give Handwritten answerarrow_forwardPlease don't provide handwritten solution ...arrow_forwardUsing the Debye-Huckel limiting law equation, Please compute ( not qualitatively) the hydroxide ion activity in a 0.050M LiOH solution? Qualitatively , it is 0.05M, I would like to see the calculations pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY