
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
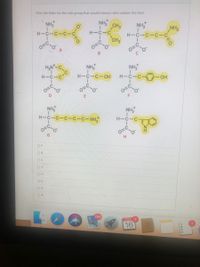
Transcribed Image Text:Give the letter for the side group that would interact with cations the best.
NH,
CH3
H-C-C
NH,
NH,
NH2
H-C-C-C-C
H-C-C- C-C
CH3
O.
HAN-CC
NH,
NH,
H-C-c
H-C-C-0H
H-C-C
OH
D
NH
H-C-C-C-C-C-NH,
NH,
O F
O B
OG
OD
284
DEC 2
16
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Bonus: Consider the molecule below. BY AN 10 Are Chloro groups A and B Geminal or vicinal to eachother? What about B and D?arrow_forward8. 3. Which of these two drawings is the best choice for an AX4E (this is a variation of an AX5)? Explain your reasoning. 부 269arrow_forwardIn point form, explain why a thiol is added to Methanearrow_forward
- 3. Explain how LDPE and LLDPE differ in terms of structure, synthesis and properties.arrow_forwardA student was attempting to prepare chiral amine with the structure shown below. After numerous attempts, each sample turned out to be optically inactive. Briefly explain why the synthesis was unsuccessful. N..arrow_forwardThe photo dimerization of benzophenone to benzopinacol is initiated by what type of electronic transition that then rapidly decomposes to a diradical since putting in electrons in anti bonds breaks bonds! The diradical then starts abstracting hydrogens from solution as pictured in the text? σ = electrons in sigma bonds n = electrons in non-bonding orbitals π electrons in pi bonds. anti bonds Hint: This process is very important because although the molecule responsible for human vision (retinal) is not a very long conjugated pi system, this transition allows retinal to absorb visible light. a. n to σ* b. σ to σ* C. π το π d. n to П e. σ to П* f. π to σ'arrow_forward
- Give the curved arrow for the structure and predict the product H*arrow_forwardO Use the polygon rule to draw the MO energy diagram of the cyclononatetraenyl anion. Assuming planarity, would this ion be aromatic or antiaromatic?arrow_forward2.How much excess acid uwos used to make the 'nitrous with the primary amine! and acid"“ HOND that will make the diazo saltarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY