
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Ff.117.
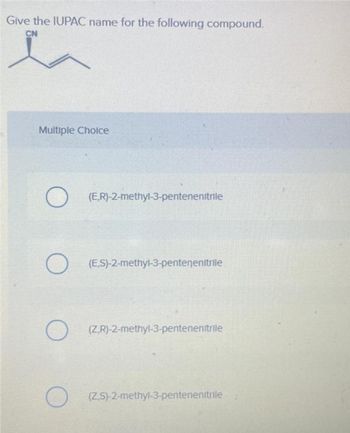
Transcribed Image Text:Give the IUPAC name for the following compound.
CN
Multiple Choice
O
O
O
O
(E,R)-2-methyl-3-pentenenitrile
(ES)-2-methyl-3-pentenenitrile
(ZR)-2-methyl-3-pentenenitrile
(ZS)-2-methyl-3-pentenenitrile
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10 Ethylene (CH,CH,) is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about 8.0 × 10º kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane (CH,CH,) from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas. Suppose an engineer studying ethane cracking fills a 60.0 L reaction tank with 22.0 atm of ethane gas and raises the temperature to 500. °C. She believes K =0.050 at this temperature. Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. Round your answer to 2 significant digits. Note for advanced students: the engineer may be mistaken about the correct value of K , and the mass percent of ethylene you calculate may not be what she actually observes. %arrow_forward1. More than 2000 years ago human cultures figured out a way to produce iron metal from rocks containing iron ores. This iron could be worked by a blacksmith (repeated heating and hammering) to make iron metal pure enough for creating useful tools (even Samurai swords). This direct heating technique was common up until about 200 years ago when people found a better way to obtain iron metal. When hematite, Fe2O3(s), is strongly heated in a blast furnace in the presence of charcoal (carbon), pure iron metal is obtained. Fe2O3() 2 Fe(s) + 3/2 O2 (g) (1) C(s) + O2 (g) → CO2(g) (2) When reaction 1 is coupled to reaction 2, overall chemical equation is Fe2O3() + 3/2 C() 3/2 CO2(g) + 2 Fee) Use the thermodynamic data given below for the following calculations: AG¡ (kJ/mol) | 4H¡ (kJ/mol) | S (J/mol-K) Fe2O3(s) -742.2 -824.2 87.40 Fes) 27.28 CO2(8) -394.36 -393.51 213.74 C (6) 5.74 O2 (2) 205.14 a) Calculate the standard Gibb's free energy change for reaction 1 b) Calculate the standard Gibb’s…arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 53.0mL of dioxygen gas are produced by this reaction, at a temperature of 50.0°C and pressure of exactly 1atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward
- Mass of crucible and cover 12.73g 2. Mass of crucible, cover, and sample 24.11g 3. Mass of original sample 24.11- 12.73g= 11.97g 4. Mass of crucible, cover, and sample after 1st heating 20.01g 6. Mass of crucible, cover, and sample after 2nd heating (assume that this step was done) 19.98 g 7. Total mass lost by sample during heating what is the percentage of water in the sample? and what is the total mass lost by the sample?arrow_forwardThe great French chemist Antoine Lavoisier discovered the law of conservation of mass in part by doing a famous experiment in 1775. In this experiment they found that mercury(II) oxide, when heated, decompose into liquid mercury and an invisible and previously unknown substance: oxygen gas. A. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury (II) oxide (HgO) into liquid mercury and gaseous dioxygen. B. Suppose 72 mL of dioxygen gas are produced by this reaction, at a temperature of 120°C and pressure of exactly 1 atm. Calculate the mass of mercury (II) oxide that must have reacted in grams. Round your answer to three significant digits.arrow_forwardWhich of the following chemical reactions is (are) classified as a decomposition reaction? A. 2NACIO3(s) 2NACI(s) + 302(g) > B. KCI(aq) + AgNO3(aq) →KNO3(aq) +AgCl(s) C. Fe(s) + CuS04(aq) FeSO4(aq) + Cu(s) D. 2NaHCO3(s) NazCO3(s) + CO2(g) + H2O(g) E. 2Fe(s) + 3C12(g) →2 FeCl3(s) A none of these B.arrow_forward
- ||| O CHEMICAL REACTIONS Balancing chemical equations with interfering coefficients Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. CH,CH, (g) + O,(g) → CO,(g) + H,O(g) X Śarrow_forward(3) A container holding NO2 and CO gas will lose NO2 gas via the reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) A chemist measures the [NO2] over time and finds that plotting 1/[NO2 ] versus time yields a straight line with a slope of 3.56×10-2 M-1·s-1. If the container originally held 1.00 M NO2, what is the concentration of NO2 after 1.50 hour [NO2]1.5 hrs = ___________ How long will it take the [NO2] to drop to 20% of its initial concentration of 1.00 M? T20% = __________arrow_forwardThe great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II) oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II) oxide (HgO) into liquid mercury and gaseous dioxygen. 2. Suppose 71.0 mL of dioxygen gas are produced by this reaction, at a temperature of 50.0 °C and pressure of exactly 1 atm. Calculate the mass of mercury(II) oxide that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY