
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give a clear explanation handwritten answer
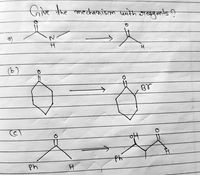
Transcribed Image Text:(b)
टा
Give the mechanism with reagents ?
H
Br
ph
N
21
H
H
ph
O.
oH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tonic water (20.0 mL) was added into each of two volumetric flasks (100 mL). The first flask was made up to volume with deionised water and produced an absorbance of 0.200. To the second flask was added an aliquot (20.0 mL) of a quinine standard solution (20.0 ppm) and after being made up to volume this produced an absorbance of 0.400. What is the concentration of quinine in the tonic water? a. 8.00 ppm b. 800 ppm С. 40.0 ppm d. 400 ppm е. 20.0 ppmarrow_forwardA standard reference material is certified to contain 94.6 ppm of an organic contaminant. Your analysis gives you the follow values, 98.6, 98.4, 95.1 and 97.2 ppm. Can you say with 95% confidence that your values differ from the certified value. Please show workarrow_forwardI need help finding the %Composition for %cychohexane and %toluene. The formula (area of peak/total area) * 100%arrow_forward
- 6-9 and 11-17arrow_forwardas X Clas X ||| STE X © Ban X Jen Scho × Ban X CD www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNsikr7j8P3jH-liG_IZvpRqwiHv-fgOzocXR7H3QULJsrn-hH7iM_OKtS081EM1kmGkq29c dent Bookmarks 00 Duolingo - The worl... ▸ YouTube Mary G. Ross: Who... Zomberry Hero > Try Again Cha X O MATTER Finding the side length of a cube from its volume in liters Your answer is incorrect. 0.75 m Trial X Explanation A technical machinist is asked to build a cubical steel tank that will hold 475 L of water. Recheck 198 X Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. → # X $ " % d H 6 H Copy of Cause and... FORENSIC SCIENC... kh hp Bay x M & 7 Ⓒ2023 McGraw Hill LLC. All Rights Re C Cop X * 00 8 ( 9arrow_forwardWith the image attached please do the calculations/calculate what is highlighted in yellow please Please also calculate - percent % error in molar mass - percent % error in pKa Please please please answer as fast as possiblearrow_forward
- only assign peaks from 1500 to 2000 cm-1arrow_forwardX A ALEKS-Allie Fleming - Knowled X + eks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9Qlgg70JQ0BkW_S727IDRdmdWICDt8DxedZZpvVEqH-ZDXitxxGhUuDfQ-ZKhas?1 # * 3 E D E Module Knowledge Check Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H₂O). What is the theoretical yield of water formed from the reaction of 2.4 g of hydrobromic acid and 0.48 g of sodium hydroxide? Round your answer to 2 significant figures. 08 I Don't Know Submit LIZ $ 4 R F 15 % 5 ▬▬ M 16 T G 0.9 6 Q Search X 17 4+ Y H & 7 Ś fa 144 U * fo 8 DII Question 5 - hp fo ( DDI 9 Al J K fm O () © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility L 112, insert P { prt sc [ با = () ← } 1 Allie V J BEEN delete olo Ar backspace h enarrow_forwardOften the analyst will compare known quantities of analyte to unknown quantities of the material to be analyzed. This may be done in one of three ways: -use calibration curves; use standard additions; or use internal standards. The method of standard additions would be used when A) the quantity of sample analyzed or the instrument response varies from run to run. B) the standard solutions and the unknown solution all have similar characteristics are unaffected by the other material in the sample. C) the sample composition is unknown or complex and affects the analytical signal.arrow_forward
- We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardPlease create a caption for this table. Solution NaCl Conc. (%) Osmolality (mOsm) % transmittance Absorbance % hemolysis % crenation C distilled 0 0 0.001029 4.987584625 100 0.03354 1 0.177179111 54.61 0.001551 4.809388202 96.42720001 0.05837 2 0.297126222 91.58 0.01012 3.994819487 80.09527231 0.08444 3 0.442542222 136.4 3.849 1.414652089 28.3634704 0.134 4 0.590164444 181.9 64.8 0.188424994 3.777880643 0.2125 5 0.74752 230.4 95.64 0.019360433 0.388172513 0.3368 6 0.89644 276.3 99.56 0.001915112 0.038397585 0.5336 7 1.095648889 337.7 99.98 8.68676E-05 0.001741676 0.9834 8 1.336711111 412 100 0 0 2.1 9 1.755568889 541.1 100 0 0 7.9 10 2.674395556 824.3 100 0 0 57.83 11 4.490211111 1384 100 0 0 99.72arrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY