
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.
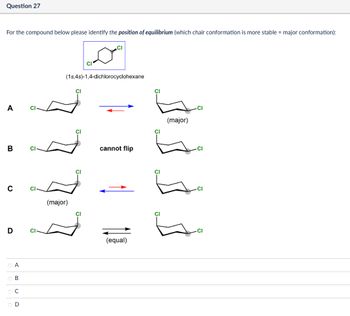
Transcribed Image Text:Question 27
For the compound below please identify the position of equilibrium (which chair conformation is more stable = major conformation):
CI
CI
(1s,4s)-1,4-dichlorocyclohexane
A
B
C
(major)
D
ABCD
○ A
OB
Ос
OD
CI
CI
CI
cannot flip
CI
(major)
CI
CI
Cl
(equal)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardDrug G is available as a weak acid (WA) and has two salt forms. Their properties are listed below: Log Ko/w Aqueous solubility Very slightly soluble Slightly soluble Sparingly soluble Drug G forms MW 416 Da | 3.3 460 Da | 2.8 Potassium Salt 520 Da | 1.9 WA Sodium Salt Drug G is available in the following formulations: Oral Solution Oral Suspension Immediate release tablet Based on the information provided, answer the following questions: 20. Which form of the drug is present in the Oral solution dosage form and why? 21. Which dosage form has the fastest onset of action? 22. Which dosage form has the smallest particle size? 23. Which dosage form will have the highest patient compliance?arrow_forward100 g of soil is leached with a strong solution of Calcium chloride such that all the exchange sites are occupied by Ca2+. The soil is subsequently leached again with a strong solution of magnesium chloride. It is determined that the resulting 100 mL leachate contains 5000 mg of Ca2+. What is the soil CEC (cmolc/kg)? The atomic wt. of Ca is 40 g/mol.please answer in word don't image upload thank you.arrow_forward
- To ensure quality solutions, follow these steps: ✔️Submit correct and complete solutions. ✔️Provide step-by-step detailed explanations. ✔️Organize your solution in a clear and structured manner. ✔️Highlight key points and important steps. ✔️Address common points of confusion. By adhering to these guidelines, You can deliver high-quality solutions that are accurate, comprehensive, and easy to understand.arrow_forwardPayalbenarrow_forwardurses/55527/quizzes/401369/take → # 3 Complete the data table using the calculation process you used to complete the previous question. Be sure to keep a copy of the completed table to include in the lab report for this experiment. $ Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 4 1.00 M Acetic Acid volume HC₂H3O2 25.0 mL 25.0 mL 25.0 mL 25.0 mL 25.0 mL Q Search f5 % moles 5 f6 Mole Ratio 6 HC₂H3O2 : NaHCO3 3:1 2:1 *All values should contain three (3) significant digits. 1:1 1:2 1:3 U hp NaHCO3 Molar Mass: 84.007 g/mol moles fg * 99+ 8 a fg mass needed DO 9 f10 ► 11arrow_forward
- 7arrow_forwardA standard reference material is certified to contain 94.6 ppm of an organic contaminant. Your analysis gives you the follow values, 98.6, 98.4, 95.1 and 97.2 ppm. Can you say with 95% confidence that your values differ from the certified value. Please show workarrow_forwardwww-awn.aleks.com ALEKS - Brittney Ortega - Learn Answered: A major component of gasoline is octane... | bartleby CHEMICAL REACTIONS Brittney V Solving for a reactant using a chemical equation Green plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water (H,O) and carbon dioxide (CO,) chemically react to form the simple sugar glucose (CH,1206) and oxygen gas (02). What mass of oxygen gas is produced by the reaction of 3.7 g of water? Round your answer to 2 significant digits. alo g Ar х10 Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibilityarrow_forward
- MBA Corp plans to use its idle building that can potentially be rented for $15,000 per annum to set up a manufacturing machinery there. The current Revenue of MBA Corp is $500,000. If the company takes up this project, its revenue is expected to increase by 30% for the next three years and then double (from the 3rd year level) for the next two years. After 5 years, the project will be scrapped and the salvage value is expected to be $80,000 The COGS are expected to remain the same at 60% of revenue. The SG&A and other operating costs will increase by $10,000. The cost of the machinery is expected to be $250,000. The machinery installation cost is expected to be $20,000. This investment will require additional inventory of $40,000 and increase the accounts payable by $20,000 The company spent $ 5000 in researching the viability of the building for machine installation. The company hires you as a financial manager to advise if they should take up this project or not. Other information:…arrow_forward3. Suppose you are to measure the BOD removal rate for a primary wastewater treatment plant. You take 2 samples of raw sewage on its way into the plant and two samples of the effluent leaving the plant. Standard 5-day BOD tests are run on the four samples, with no seeding producing the following data: Sample Dilution Doi (mg/L) DOf (mg/L) Source 1 Raw 1:30 9.0 2.2 2 Raw 1:20 9.0 ? 3 Treated 1:15 8.5 1.5 4 Treated ? 9.0 1 a. Find the BOD5 for the raw and treated sewage, and the percent removal of BOD in the treatment plant. b. Find the DO that would be expected in sample 2 at the end of the test. c. What would be the maximum volume of treated sewage for sample 4 that could be put into the 300 mL BOD bottle and still have the DO after 5 days remain above zero?arrow_forwardThe three major steps in the QA process are Use objectives, fortification, blanks Use objectives, specifications, assessment Use objectives, false positives, raw data Fortification, blanks, calibration testing Raw data, treated data, resultsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY