
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Complete the following reactions:
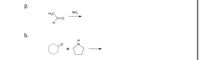
Transcribed Image Text:g.
H3C
NH,
h.
H.
N.
Expert Solution
arrow_forward
Step 1
Most of the ketones and aldehydes react with 2º (secondary)-amines to give the compounds known as the enamines. In this reaction, there are acid-catalyzed reversible reactions where water (H2O) is lost. Subsequently, the enamines can be easily converted back to their corresponding carbonyl precursors using acid-catalyzed hydrolysis.
The ketones and aldehydes react with 1º (primary)-amines to give the compounds known as the imines or the Schiff bases, that is, the compounds having a C=N (carbon-nitrogen) function. H2O is removed in the reaction.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw (S)-3-hydroxy-2,2,4-trimethylpentanalarrow_forwardWhat is the structural formula of ethyl 3-methylbutanoate? A CH,CH,COOCH,CH,CH(CH,), B CH,CH,COOCH(CH,)CH,CH, C CH,CH,CH(CH,)COOCH,CH, D (CH,),CHCH,COOCH,CH,arrow_forwardClassify each reaction as addition, elimination, substitution, rearrangement, oxidation, or reductionarrow_forward
- According to Markovnikov's rule when HI (hydrogen iodide) adds to H3C-CH2-HC=CH2,, the main product is 2-iodobutane 1-iodobutene 2-iodobutene 1-iodobutanearrow_forwardWhat alcohol is formed when the alkene CH3CH2CH=CH2 is treated with H2O in the presence of H2SO4?arrow_forwardDraw the organic product(s) formed upon the addition of HBr to (a) 2-methyl-2-pentene, (b) trans-2-hexene, and (c) 4-methylcyclohexene. How many regioisomers can be formed in each case?arrow_forward
- When propanol (C₃H₇OH) is burned in excess oxygen, what is/are the product(s) of this reaction?arrow_forwardThe following compound can be prepared by a Claisen condensation followed by saponification and decarboxylation. Propose a structural formula for the ethyl ester precursor that undergoes a Claisen condensation. Нarrow_forwardName the alcohol that is formed when the following alkene undergoes hydration by water in the presence of a small amount of sulfuric acid:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY