
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
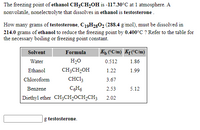
Transcribed Image Text:The freezing point of ethanol CH3CH,0H is -117.30°C at 1 atmosphere. A
nonvolatile, nonelectrolyte that dissolves in ethanol is testosterone.
How many grams of testosterone, C19H2802 (288.4 g/mol), must be dissolved in
214.0 grams of ethanol to reduce the freezing point by 0.400°C ? Refer to the table for
the necessary boiling or freezing point constant.
Solvent
Formula
Kh CC/m) Kf(°C/m)
Water
H2O
0.512
1.86
Ethanol
CH;CH,OH
1.22
1.99
Chloroform
CHC13
3.67
Benzene
CH6
2.53
5.12
Diethyl ether CH;CH2OCH2CH3
2.02
g testosterone.
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could you please solve these two and also this question below? which of the following is a disaccharide? a. Fructose b. Galactose c. Maltose d. Amylose e. Glucosearrow_forwardWhat is the difference between flaming combustion and glowing combustion?arrow_forwardWhat is the name of the carbohydrate compound C? но H. CH2OH C A. D-xylose В. D-lyxose C. L-threose D. D-erythrose E. L-erythrose F. D-threose G. L-lyxose H. L-xylosearrow_forward
- In the answer boxes, indicate whether carbon 2 compound. Br ** HO **|||| H and carbon 3 hove R or 3 configuration in the followingarrow_forwardLactose is a disaccharide created by the bonding of which two monosaccharidesarrow_forwardClassify the following disaccharide. a. beta 1-2 disaccharide b. alpha 1-4 disaccharide c. alpha 1-6 disaccharide d. alpha 1-2 disaccharide e. beta 1-4 disaccharide f. beta 1-6 disaccharidearrow_forward
- Which of the following molecules is optically active? CH3 CH3 CH3 CH3arrow_forwardSeliwanoff's tests is used to distinguish a. a ketose from an aldose. b. a monosaccharide from a disaccharide. c. a polysaccharide from a disaccharide. d. a reducing sugar from a nonreducing sugar. e. a monosaccharide from a polysaccharide.arrow_forwardGlucose has which functional group?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY