
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
From the Lewis structure shown below select does it have represent the Resonance of HIO2 ( it doesn’t have to be the lowest energy structure)
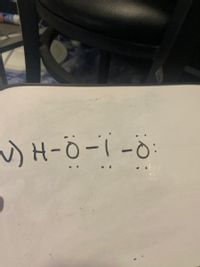
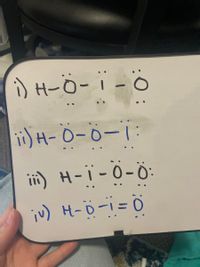
Transcribed Image Text:) H-Ö- i - ö
ii) H-0-0-1
ii) H-i - 0-Ö
iu) H-ö-1= 0
:0:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the Lewis structures for CH2N2, including all resonance forms, and show formal charges.arrow_forwardIn the Lewis structure for cyanate ion given below, what is the formal charge on the carbon atom?arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of zero. H I H-C-C-N-O | H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forward
- ng and lear X G The element in period 5 that has X C Solved The following Lewis diagr. X nt/takeCovalentActivity.do?locator-assignment-take The formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. Formal Charge 01 We can draw three inequivalent Lewis structures for carbon dioxide, CO₂. The concepts of formal charge and electronegativity can help us choose the structure that is the most significant representation. 1. Assign formal charges to the elements in each of the structures below. C [Review Topics] [References] Use the References to access important values if needed for this question. 02 :0₁-C=0₂: A 0,=C=0₂ :0,=C-0₂: + B 999 C *** 2. The structure that contributes most significantly to the overall electronic structure of CO₂ is. AG ENG neme Next 11:52 AM 11/28/2022 [arrow_forwardIn consideration of the overall resonance hybrid OCN(^-), which is the proposed resonance structure that's most important? In lewis Dot Form*arrow_forwardDecide if each of the electron dot pictures, shown below for the indicated molecules, is an acceptable Lewis structure. Lewis structure of O2? Lewis structure of CO?arrow_forward
- O ELECTRONIC STRUCTURE AND CHEMICA... Deciding whether a Lewis... Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure [O=C-H]* :0: : CIC CI: [¤¤-6: 0/5 Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. 000 The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0 Alia V No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* X Ar If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example,…arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure -C=C:] H-C : 0: 0=0=0 [HO] : O OO Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* П Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "O,0".arrow_forwardAn incompicie Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of -1. N-C-C-H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. H N=c=c-Harrow_forward
- Give a clear handwritten answer with explanationarrow_forwardThe formal charge is the "charge" an element would have in a molecule or ion if all of the bonding electrons were shared equally between atoms. We can draw three inequivalent Lewis structures for the thiocyanate ion , SCN" . The concepts of formal charge and electronegativity can help us choose the structure that is the most significant representation. 1. Assign formal charges to the elements in each of the structures below. :S- C=N: S =C=N :S= C-N: А В Formal Charge C N 2. The structure that contributes most significantly to the overall electronic structure of SCN¯ isarrow_forwardCaffeine is a stimulant, and is the fuel that powers lots of early mornings and late night study sessions. The molecular formula of caffeince is CgH,,N402. The skeletal structure is shown below. Complete the Lewis structure for our good friend caffeine. Q H to [] H NS P Br CI Morearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY