
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
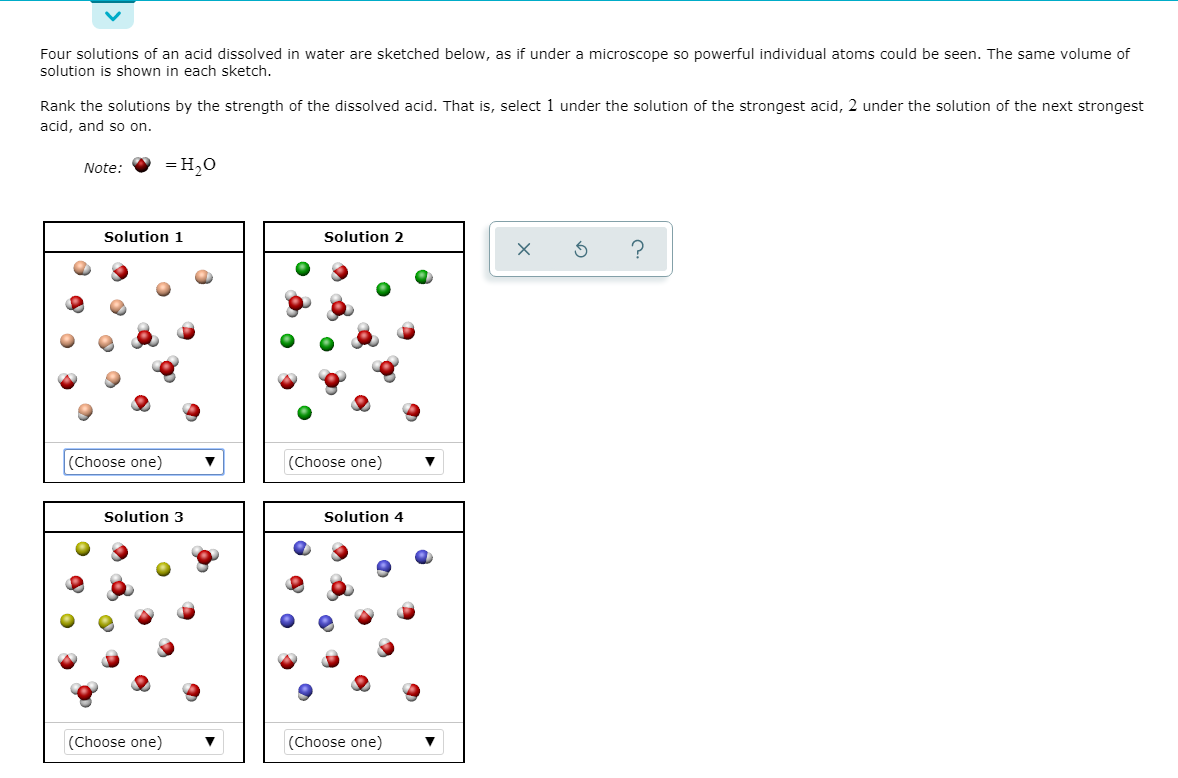
Transcribed Image Text:Four solutions of an acid dissolved in water are sketched below, as if under a microscope so powerful individual atoms could be seen. The same volume of
solution is shown in each sketch.
Rank the solutions by the strength of the dissolved acid. That is, select 1 under the solution of the strongest acid, 2 under the solution of the next strongest
acid, and so on.
Note:
= H,0
Solution 1
Solution 2
hoose one)
(Choose one)
Solution 3
Solution 4
(Choose one)
(Choose one)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- Assuming complete dissociation, what is the pH of a 4.04 mg/L Ba(OH)2 solution? pH=arrow_forwardWhich solution below has the highest concentration of hydroxide ions? pH = 8.2 pH = 5.6 pH = 3.7 pH = 11.0 pH = 11arrow_forwardA particular solution has a [OH^−1] = 8.31×10^−5 . Calculate the [H+1]for this solution. A) 1.20×10^−10 B) 7.36×10^−10 C) 8.31×10^9 D) 3.18×10^−11 E) 2.62×10^−10arrow_forward
- Consider a solution which is [OH-] = 8.4 x 10-11 M. Calculate the [H3O+].arrow_forwardAssuming complete dissociation, what is the pH of a 4.12 mg/L Ba(OH)₂ solution? pH =arrow_forward1a) The hydronium ion concentration of an aqueous solution of 0.394 M diethylamine (a weak base with the formula (C2H5)2NH) is[H3O+] = ______ M. b) The pOH of an aqueous solution of 0.394 M ethylamine (a weak base with the formula C2H5NH2) is ______arrow_forward
- According to the Bronsted Lowry definition of an acid, in the reaction below, HC,H3O2 is considered an acid because-- HC2H3O2 → H* + C2H3O2 O It accepts a proton. O Increases the hydroxide ion concentration. O It donates a proton. O It increases the hydrogen ion concentrationarrow_forwardWhich of the following shows the correct relationship between the ions in aqueous solutions at 25°C? ) [H30*1×[OH¯] = 1.0 x 10-7 2) [H,o"] + [OH] = 1.0 x 10-14 3 [H30"]< [OH] = 1.0 x 10-14 4 [H,0"] ÷ [OH] = 1.0 x 10-14 5 [H30"] x [OH] = 1.0 x 10-14arrow_forwardWhat is an acid-base reaction? Match the items in the left column to the appropriate blanks in the sentences on the right. a precipitate a new acid or a new base OH- H₂O(1) H+ When an acid and base are mixed, the from the base to form from the acid combines with the Reset Helparrow_forward
- Each row of the table below describes an aqueous solution at about 25 °C. Complete the table. That is, fill in any missing entries in the second and third columns. Round your entries for H,O' to 2 significant digits, and your entries for pH to 2 decimal places. solution pH K10 A 6.2 x 10 mol/L |mol/L 8.48 7.8 x 10 mol/Larrow_forward3arrow_forwardНА А- - H+ HB НА H* A- HB HB H* B- B- Figure 1 Figure 2 Which process shows the dissociation for a weak solution? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY