
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
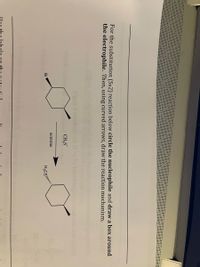
Transcribed Image Text:**Transcription and Explanation for Educational Use**
---
**Title:** Understanding the SN2 Reaction Mechanism
**Instructions:**
For the substitution (SN2) reaction below, circle the nucleophile and draw a box around the electrophile. Then, using curved arrows, draw the reaction mechanism.
**Diagram Explanation:**
1. **Reactants:**
- A cyclohexane ring is shown with a bromine atom (Br) attached as a leaving group.
- \( \text{CH}_3\text{S}^- \) (methanethiolate anion) is depicted as the nucleophile.
2. **Reaction Condition:**
- The reaction is indicated to occur in the presence of acetone, a common solvent for SN2 reactions.
3. **Curved Arrow Notation:**
- Curved arrows in organic chemistry illustrate the movement of electron pairs. In an SN2 reaction, a single step process, the nucleophile attacks the electrophile simultaneously as the leaving group departs.
4. **Product:**
- The product of this reaction is a cyclohexane with the methanethiol group \( \text{H}_3\text{S} \) replacing the bromine.
---
In this SN2 reaction, the methanethiolate anion acts as the nucleophile, attacking the electrophilic carbon atom bonded to bromine. Concurrently, bromine leaves, resulting in the substitution product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows ← Undo W Reset Ⓒ Done H H H Oarrow_forwardCircle the alkyl halide that will be the most reactive in an SN1 reaction. H CI & or CIarrow_forwardgive mechanismarrow_forward
- 4. Draw the mechanism of the following SN2 reaction and the products with Stereochemistry. Draw the transition state. CI H ENarrow_forwardQ15. Draw all the resonance forms of the cationic intermediate formed by the reaction of phenol (1) and the nitroniym ion (2). Draw resonance forms for both para and meta attack? According to the stability of the resonance forms you drew, which attack is favoured? OHarrow_forwardDraw all of the products and the mechanism for the reactions below.arrow_forward
- Draw the mechanism for the prbolem below! Make sure to include formsl charge, intermediates, and mechanismarrow_forward10. a) In the box at the left, indicate whether the reaction takes place through an SN2, SN1, E2, or E1 mechanism. b) Draw the mechanism and predict the product, including stereochemistry. OH H3O* heat CI NaCN DMF KOt-Bu Br HOH HCIarrow_forward1. For the substitution (SN2) reaction below circle the nucleophile and draw a box around the electrophile. Then, using curved arrows, draw the reaction mechanism. CH;S acetone Brarrow_forward
- Draw the mechanism of reaction A using curved arrows. The formation of an electrophile does not need to be presented, but the answer must reveal what the electrophile is.arrow_forwardIf H* is eliminated from the carbocation shown here in an electrophile elimination step, then three possible constitutional isomers can form. Draw the mechanism for the formation of all three of those products. H2O + ?arrow_forwardDraw the reaction mechanism for the following transformation given the starting materials, product,and reagents. Be sure to include all intermediates,curvy reaction arrows,and include any formal charges.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY