
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
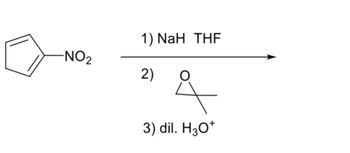
Transcribed Image Text:NO₂
1) NaH THF
2)
of
3) dil. H3O+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the balanced neutralization equation for the reaction below: HCl(aq) + Sr(OH)₂(aq) → Reset 2 3 4 5 6 7 8 ) (1) O CI Sr H Tap here or pull up for additional resources 30₂ 1 2- + (s) CO (g) 个 9 11 ( (aq) • x H₂Oarrow_forwardQuestion 83.arrow_forwardHow can I fill in this flow chart ?arrow_forward
- Ph and pOh of 0.020m HNO3arrow_forwardWill the following reaction yields a precipitate? yes or no Pb(NO3)2(aq) + NaC2H3O2(aq) ->arrow_forward4. (a) Calculate [H3O*] and [OH] for the solutions indicated: lemon juice, pH = 2.42 (1) (ii) household ammonia pH = 11.21 (iii) (iv) (v) phosphate buffer pH = 6.96 human urine pOH = 7.8 tomato juice pOH = 9.9arrow_forward
- Q18arrow_forwardWhat mass of Ba(OH)2 is present in a sample if it is titrated to its equivalence point with 44.20 mL of 0.1000 N H2SO4? Note: Present complete solutions for the following problem. Express your final answers up to two (2) decimal places.arrow_forwardA neutralization reaction between KOH (aq) and H2SO4 (aq) would give which two products?arrow_forward
- Calculate the concentration of HCO3 in an aqueous solution of 0.1120 M carbonic acid, H,CO3 (aq). [НСО3]%3D M.arrow_forwardCalculate the pH of the solution in which 0.2M NH4Cl and 0.1M NH3 are present. The pKb of ammonia solution is 4.75.arrow_forward2. Using the procedure described in this module, a student determined the percent KHP in an impure sample of KHP. A 3.150-g sample of impure KHP required 41.50 mL of 0.1352M NaOH solution for titration. (a) Calculate the number of moles of NaOH required for the titration. (b) Calculate the number of moles of KHP present in the impure sample of KHP. (c) Calculate the number of grams of KHP present in the impure sample. (d) Calculate the percent of KHP in the impure sample, using Equation 8. Equation 8: percent KHP in the impure sample, % = ( mass of KHP in the sample,g/ mass of sample analyzed, g) (100%)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY