
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution ....
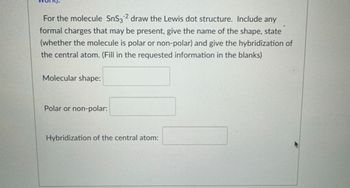
Transcribed Image Text:For the molecule SnS32 draw the Lewis dot structure. Include any
formal charges that may be present, give the name of the shape, state
(whether the molecule is polar or non-polar) and give the hybridization of
the central atom. (Fill in the requested information in the blanks)
Molecular shape:
Polar or non-polar:
Hybridization of the central atom:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Despite their ability to heal, drugs can naturally become dangerous if consumed in excessive quantities. Thus, for each drug sold, the potentially lethal dose (called dl50) was measured when ingested. For acetaminophen (Tylenol), this lethal dose is 2400 mg/kg body weight. a) Based on this value, how many 350 mg tablets is the life-threatening dose for an individual weighing 195 lbs? The conversion factor is 2.200 lbs/kg. b) Calculate Aspirin ld50 (mg/kg body weight) if 25 225 mg pills can be fatal for a 60.0 lbs child.arrow_forward5 What initial volume of a 24.00 M solution must have been present in order to allow for a 0.1000 Molar solution to have been created with a final volume of 1.800E6 mL of solution? There must have been an initial volume of type your answer... mLarrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Carbon dioxide dissolved in water to yield carbonic acid. Carbonic acidthen partially dissociates to produce the hydrogen carbonate anion and ahydrogen cation. (write two equations for this step)arrow_forwardAn aqueous solution of aluminum iodide, All3, contains 12.6 grams of aluminum iodide and 17.5 grams of water. The percentage by mass of aluminum iodide in the solution is %.arrow_forwardCombustion of hydrocarbons such as undecane (C,H) produces carbon dioxide, a "greenhouse gas. Greenhaluse gases in the Earth's atmosahere tan trap the Sun's heat, raising the averege temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carban diaxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid undecane into pmequs carbon diocide and gaeous water. D-0 2. Suppose 0.300 kg of undecane are burned in air at a pressure of exatly I atm nd a temperature of 17.0 "C. Calculate the volume of carbon dionide gas that is produced. Round your answer to 3 significant digits.arrow_forward
- What can you reasonably predict to occur when elemental bromine is added to a solution containing the iodide ion? Write chemical equations as part of your explanation.arrow_forwardAt a particular temperature, suppose that 24.4 g solute is added to 50.0 g water and, after mixing, 0.3 g of the solute remains undissolved. What is the solubility of this solute at this temperature in grams per 100 g of water? (Enter in standard notation with one digit after the decimal point) Type your answer......arrow_forwardHow do I calculate/ what is the equation for the % weight/weight when I have a mass in grams and a volume of titrant delivered? Unknown Chrloride mass 0.1173g and 35.47 ml.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY