
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
10
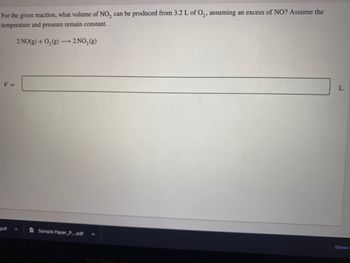
Transcribed Image Text:For the given reaction, what volume of NO₂ can be produced from 3.2 L of O₂, assuming an excess of NO? Assume the
temperature and pressure remain constant.
V =
2 NO(g) + O₂(g) →→→ 2 NO₂(g)
-
pdf A
Sample Paper_P....pdf
MacBook
L
Show A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume (in liters) of O2, measured at standard temperature and pressure, is required to oxidize 0.400 mol of phosphorus (P4)? P4(s) + 5 O2(g) P4O10(s)arrow_forwardXenon and fluorine will react to form binary compounds when a mixture of these two gases is heated to 400C in a nickel reaction vessel. A 100.0-mL nickel container is filled with xenon and fluorine, giving partial pressures of 1.24 atm and 10.10 atm, respectively, at a temperature of 25C. The reaction vessel is heated to 400C to cause a reaction to occur and then cooled to a temperature at which F2 is a gas and the xenon fluoride compound produced is a nonvolatile solid. The remaining F2 gas is transferred to another 100.0-mL nickel container, where the pressure of F2 at 25C is 7.62 atm. Assuming all of the xenon has reacted, what is the formula of the product?arrow_forwardAt elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. A 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. The oxygen gas collected over water occupied 57.2 mL at a temperature of 22C and a pressure of 734 torr. Calculate the mass percent of NaClO3 in the original sample. (At 22C the vapor pressure of water is 19.8 torr.)arrow_forward
- 105 The decomposition of mercury(II) thiocyanate produces an odd brown snake-like mass that is so unusual the process was once used in fireworks displays. There are actually several reactions that take place when the solid Hg(SCN)2 is ignited: 2Hg(SCN)2(s)2HgS(s)+CS2(s)+C3N4(s)CS2(s)+3O2(g)CO2(g)+2SO2(g)2C3N4(s)3(CN)2(g)+N2(g)HgS(s)+O2(g)Hg(l)+SO2(g) A 42.4-g sample of Hg(SCN)2 is placed into a 2.4-L vessel at 21°C. The vessel also contains air at a pressure of 758 torr. The container is sealed and the mixture is ignited, causing the reaction sequence above to occur. Once the reaction is complete, the container is cooled back to the original temperature of 21°C. (a) Without doing numerical calculations, predict whether the final pressure in the vessel will be greater than, less than, or equal to the initial pressure. Explain your answer. (b) Calculate the final pressure and compare your result with your prediction. (Assume that the mole fraction of O2 in air is 0.21.)arrow_forwardYou have a gas, one of the three known phosphorus-fluorine compounds (PF3, PF3, and P2F4). To find out which, you have decided to measure its molar mass. (a) First, yon determine that the density of the gas is 5.60 g/L at a pressure of 0.971 atm and a temperature of 18.2 C. Calculate the molar mass and identify the compound. (b) To check the results from part (a), you decide to measure the molar mass based on the relative rales of effusion of the unknown gas and CO2. You find that CO2 effuses at a rate of 0.050 mol/min, whereas the unknown phosphorus fluoride effuses at a rate of 0.028 mol/min. Calculate the molar mass of the unknown gas based on these results.arrow_forwardGold is produced electrochemically from an aqueous solution of Au(CN)2 containing an excess of CN. Gold metal and oxygen gas are produced at the electrodes. What amount (moles) of O2 will be produced during the production of 1.00 mole of gold?arrow_forward
- Methanol (CH3OH) can be produced by the following reaction: CO(g)+2H2(g)CH3OH(g) Hydrogen at STP flows into a reactor at a rate of 16.0 L/min. Carbon monoxide at STP flows into the reactor at a rate of 25.0 L/min. If 5.30 g methanol is produced per minute, what is the percent yield of the reaction?arrow_forwardYou have two pressure-proof steel cylinders of equal volume, one containing 1.0 kg of CO and the other containing 1.0 kg of acetylene, C2H2. (a) In which cylinder is the pressure greater at 25 C? (b) Which cylinder contains the greater number of molecules?arrow_forwardDescribe what happens o the average kinetic energy of ideal gas molecules when the conditions are changed as follows: (a) The pressure of the gas is increased by reducing the volume at constant temperature. (b) The pressure of the gas is increased by increasing the temperature at constant volume. (c) The average velocity of the molecules is increased by a factor of 2.arrow_forward
- The nitrogen content of organic compounds can be determined by the Dumas method. The compound in question is first reacted by passage over hot CuO(s): CompoundCuO(s)HotN2(g)+CO2(g)+H2O(g) The product gas is then passed through a concentrated solution of KOH to remove the CO2. After passage through the KOH solution, the gas contains N2 and is saturated with water vapor. In a given experiment a 0.253-g sample of a compound produced 31.8 mL N2 saturated with water vapor at 25C and 726 torr. What is the mass percent of nitrogen in the compound? (The vapor pressure of water at 25C is 23.8 torr.)arrow_forward52 If tetraborane, B4H10, is treated with pure oxygen, it burns to give B2O3 and H2O: 2B4H10(s)+11O2(g)4B2O3(s)+10H2O(g) If a 0.050-g sample of tetraborane burns completely in O2, what will be the pressure of the gaseous water in a 4.25-L flask at 30.0 C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning