
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
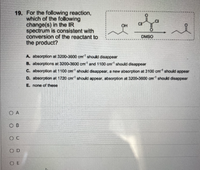
Transcribed Image Text:19. For the following reaction,
which of the following
change(s) in the IR
spectrum is consistent with
conversion of the reactant to
the product?
DMSO
A. absorption at 3200-3600 cm should disappear
B. absorptions at 3200-3600 cm and 1100 om should disappear
C. absorption at 1100 cm should disappear, a new absorption at 3100 cm should appear
D. absorption at 1720 cm should appear, absorption at 3200-3600 cm should disappear
E. none of these
O A
O B
O D
O E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The product of the reaction in the box has five signals in the 13C NMR. What is the structure of that product? A) C) Br Br. Br Br₂ B) D) Br Bri ? Br Brarrow_forwardFor the following reaction, what significant changes would be expected by IR absorptions, (ignoring C-H absorption): * a. CH;MgBr HO. b. H;0 A peak around 3300-3500/cm would appear O A peak around 1710/cm would appear No change observed. A peak around 3300-3500/cm would disappeararrow_forwardI,m not sure how to answer this because they are both C-H bonds so they have the same mass. I thought the stronger the bond, the higher the wavenumber? how does wavenumber relate to energy?arrow_forward
- Imagine that you were given an unidentified aldehyde and performed another Wittig reaction in lab. Use the given data to answer the questions below and identify your original aldehyde. 8 5 R A H 7 + 15 1 C/O (C6H5)3P- Below is shown the ¹H spectrum for the pure alkene product of this experiment. Interpret the signals to identify "R" by assigning each hydrogen by chemical shift, multiplicity (splitting), integration, and any other significant features. 6 A B -5 NaOH 4 PPM (b) One possible alkene product is shown in the reaction above. Draw the other alkene product R 3 H 2 + (C6H5)3PO 2 1 6 1 (a) Label which proton in the product above corresponds to each signal A and B. Explain your assignment and what this tells you about the "R" group. (c) What was your aldehyde starting material? (What is "R"?) 1arrow_forwarda) classify the amines shown as either primary, secondary, or tertiary based on the number of carbons attached to the nitrogen atom b) what is different in the IR spectra of primary, secondary, and tertiary amines? Is this a way to distinguish them? Explain c) explain how to differentiate amines and alcohols via IR spectral analysisarrow_forwardTo preview image click here Br Br & 1 11 A. How many signals would you expect to see in the ¹HNMR spectrum of each of the following compounds. 1. For Structure | [Select] 2. For Structure II [Select] B. How many signals would you expect to see in the 13CNMR spectrum of each of the following compounds. 1. For Structure | [Select] 2. For Structure II [Select ] H >arrow_forward
- LAB Q4. Draw the structure of the synthesized product (do not include side or by products). How can IR spectroscopy be used to monitor the progress of each of the following reactions (if reaction went to completion and/or check the purity of the final product)? What absorptions would be observed in the IR spectrum? And if IR spectroscopy could not have been used, briefly explain why.arrow_forwardCan you please explain the stepsarrow_forwardAssigning Spectra: The peak at 0.9 ppm is a doublet, integrating to 6. Assign this peak to a fragment (Which statement is most correct?) * 20 10 ppm R2-CH-CH2-C(=0)-OR. Based on shift it could be a CH2 near carbonyl, with one neighboring proton. R2-CH-CH3. Could be a standard methyl, with one neighboring proton. O R-CH2-CH3. Could be a standard R-CH3, with 2 neighboring protons. RO-CH(R)-CH3. Could be standard methyl, with beta substituent, and one neighboring proton.arrow_forward
- Predict the splitting pattern for protons labeled a, b, and c, giving that for "a" first. b C CI-CH₂-CH₂-O-CH3 in order of protons a, b, c. a A. triplet, triplet, singlet B. multiplet, triplet, singlet OC. all singlets OD. triplet, multiplet, triplet O E. doublet, doublet, singletarrow_forwardWhat cations are formed in the mass spectrometer by α cleavage of each of the following compounds?arrow_forward2. READ THE DIRECTIONS TO EACH QUESTION CAREFULLY - not following the directions means you get the question wrong! 3. FORMAT YOUR ANSWERS AS DIRECTED - formatting your answers incorrect means you get the question wrong! 4. Below are the IR regions and NMR chemical shifts table for use with the spectroscopy problems: ¹H NMR 12 R ΟΞΟ 11 10 PIC OH R H 9 8 7 ppm 6 OH H C=C-H 5 NH H-C-X H-C-O -OH X = F, Cl, Br (i.e. electronegative atom) 3 -NH 2 H-C-N H-C-S 1 -CH3 -CH₂- C=C-H H-C-C=O -CH H-C-C=C HC-0 0 4000 small range Hrange of values broad peak DID =C-H N-H 3250- 3300 broad with spikes -3300 3500 -O-H broad-3300 -3400 -O-H CEN usually strong N-H CIO C H ₹ -C-H O 2730- 2820 3000- 2 peaks 3100 2850- 2960 O -C-O-H broad-3000 3000 H -CEN 2500 wavenumber, cm-1 2200 <-CICH B 2200 2000 CC H.C. N 1680 000000 H 1600- 1660 0 OR 1730 1710 1600 NR₂ 1650 1500 1000arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY