
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
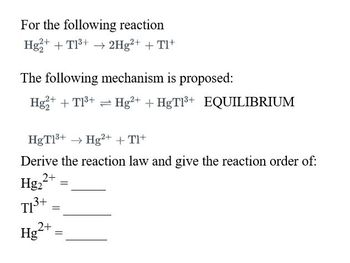
Transcribed Image Text:For the following reaction
Hg2+ + T1³+ → 2Hg²+ + Tl+
The following mechanism is proposed:
Hg2+ + T1³+ Hg²+ + HgT1³+ EQUILIBRIUM
HgT1³+ → Hg2+ + Tl+
Derive the reaction law and give the reaction order of:
Hg₂2+
T1³+
3+ =
2+
Hg
=
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help, with detailed explanation need. Need correct solution only.arrow_forwardPlease helparrow_forwardThe following data was obtained for the decomposition of X2 in the gas phase. X2→Y + Z 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 20.00 (hr) [X2] 0.250 0.213 0.182 0.155 0.133 0.113 0.097 | 0.082 0.070 0.060 0.051 a. Determine the rate constant for this reaction. b. How much X2 is left if 0.457 M of the gas is allowed to decompose for 1.75 hours? c. What is the half-life of 0.892 M X2?arrow_forward
- (2) The reaction-rate data given below were obtained using a batch reaction system for the reaction A→products. Plot the data in appropriate form to test the fit for zero- and first-order kinetics. From the plot that gives the best fit, determine the appropriate reaction rate constant (remember the units). Time, min CA, (g/m3) 0 30 0.5 25.4 1 21.6 2 16.9 4 13.1 8 8.3 16 3.2 32 0.31arrow_forwardGivena reaction 2A + B---> C + 2D which is found to be second order in a and independent of B, is the proposed slow step of the mechanism 2A--> D + E consistent with the experimental data? I don't think so, but i would like confirmation.arrow_forwardDETERMINATION OF A,H FOR THE REACTION OF Mg AND HCl Minimize D The results from one run are shown below. 40 00003333333t1&C66C0000000000333333113ttteeccc000000 y = -0.51215 x + 40.274 20 10 4 5 Time (min) Q3 Use the graph to determine Tinitial and Tfinal. You must then use these values to calculate the change in temperature for the reaction. Tinitial (°C) Tinal (°C) AT (°C) 1 2 Temperature (°C) 30arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY