
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
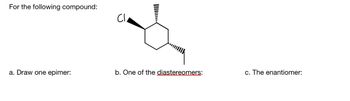
Transcribed Image Text:For the following compound:
a. Draw one epimer:
b. One of the diastereomers:
c. The enantiomer:
Expert Solution
arrow_forward
Step 1: Stereoisomer
The molecules with the same molecular formula but different structures are known as isomers. If the two molecules have the same molecular formula and the same bond connectivity but the arrangements of the atoms in space are different, are stereoisomers of each other.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help!arrow_forwardThe two enantiomers of a chiral compound are present in the ratio 50:25. What is the enantiomeric excess for this compound? Select one: а. 100% O b. None of the others С. 50% d. 25% е. 20%arrow_forwardC 23. a) The observed rotation for a solution containing the R and S enetaiomers a of a sample was +17.6. An enantiomerically pure sample of the S enantiomer has a specific rotation of + 22.3°. What is the % enantiomeric excess of sample A? b)A pure sample of the R-enantiomer of a compound has a specific rotation of -15°. A solution containing 0.6 g/mL of a mixture of enantiomers rotates plane polarized light by -3° in a 1 dm polarimeter. What is the enantiomeric excess (%ee) of the mixture? 9arrow_forward
- Finding and Drawing Stereogenic Centers 5.45 Locate the tetrahedral stereogenic center(s) in each compound. A molecule may have one or more stereogenic centers. • stereogenic centers a. 어 I tetrahedral stercogenic center. b. C. d. OH OH OH OH OH OH ملمه D OH e. HO HO f. OH OH OHarrow_forwardCI CI Compaund A Compund B a) draw the enantiomers of Compound A and Compound B if possiblearrow_forwardWhat is the percent enantiomeric excess (ee) of a mixture that has 86% of one enantiomer and 14% of the other?arrow_forward
- CI CI a b .CI d Provide the name for each of the above compounds, and determine if the compound is chiral. (Do not include any stereochemical designations in the name. If a box is not needed, leave it blank.) a. Name: Chiral? b. Name: Chiral? c. Name: Chiral? d. Name: Chiral?arrow_forwardHow many stereoisomers are possible for the following: Select one: O a. sixteen O b. four C. two O d. eight OH OHarrow_forwardA. Draw the enantiomer of the following compound. B. Draw a diastereomer of the following compound. „CI H. H2N"arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY