
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
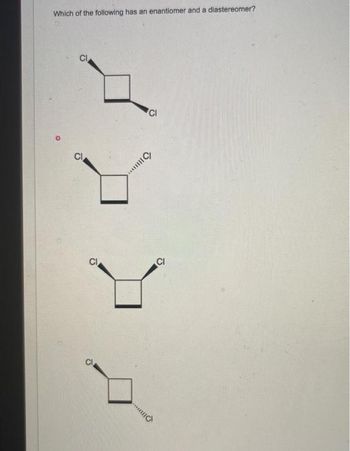
Transcribed Image Text:Which of the following has an enantiomer and a diastereomer?
CI
...C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- draw the enantiomers' and diastereomers of the following compunds.arrow_forwardPlease describe as either constitutional isomers, same compound, enantiomers or diastereomers.arrow_forwardWhich of the following is the definition of a pair of enantiomers? a. A pair of structures that are superposable mirror images of one another b. A pair of stereoisomers that are non-superposable mirror images of one another c. A pair of stereoisomers that are not mirror images of one another d. A pair of stereoisomers that have equal specific rotations Group of answer choices a b c darrow_forward
- What is the relationship between the molecules shown? HO. HO same molecules constitutional isomers diastereomers O enantiomers ......arrow_forward5. What is the relationship between the following 2 molecules HOI a Enantiomers b.) Diastereomers Identical d. Not isomers OH K ...arrow_forwardPlease draw the fischer projections for the following compound and determine whether it is (R) or (S) Thank youarrow_forward
- Part A A weather balloon is inflated to a volume of 25.5 L at a pressure of 738 mmHg and a temperature of 31.4 °C. The balloon rises in the atmosphere to an altitude where the pressure is 390. mmHg and the temperature is -15.6 °C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude. a) ? Vballoon = L Submit Request Answer What is the pressure if the volume of the container is maintained constant and the temperature is raised to 331 °C? Express the pressure in atmospheres to three significant figures. ΑΣφ. P = atm Submit Request Answer What is the density (in g/L) of hydrogen gas at 20 °C and a pressure of 1655 psi? Express your answer in grams per liter to three significant figures. • View Available Hint(s) Πνα ΑΣφ d = g/L Submitarrow_forwardWhich of the following is a DIASTEREOMER of OA B oc OD HO OH OH OH HO OH ÓH B Darrow_forward3. Label any stereocenters in the following molecule as (R) or (S): CIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY