
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
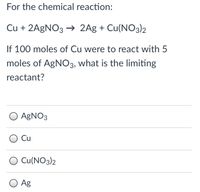
Transcribed Image Text:For the chemical reaction:
Cu + 2A9NO3 → 2Ag + Cu(NO3)2
If 100 moles of Cu were to react with 5
moles of AGNO3, what is the limiting
reactant?
O AGNO3
Cu
O Cu(NO3)2
Ag
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following chemical reaction, be sure to include “1” in the blank for any compounds with a stoichiometric coefficient of 1. Li(s) + H2O(l) → LiOH(aq) + H2(g)arrow_forwardThe following precipitation reaction is perfomed using 3.785 g of FeCk and 2.258 g of NaOH. FeCk (aq) +3 NaOH (aq) Fe(OH): (s) 3 NaCl (ag) a. What is the theoretical yield of the produce (solid) in this reaction? b. If the reaction produces 1.825 g of the product, what is the percent yield? c. Write the full and net ionic equations for this reaction.arrow_forwardAn alloy is found to be 41.8% Zn and the rest is Cu. If 15.0 g of Cu is added to 45.0 g of This alloy , what will be the new percentage of Cu in the alloy ?arrow_forward
- Combining 0.266 mol Fe,O, with excess carbon produced 20.0 g Fe. Fe, O, + 3 C → 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: molarrow_forwardThe equation for one of the reactions in the process of turning iron ore info the metal is Fe2O3 + 3CO --> 2Fe + 3CO2 If you start with 2.00 kg of each reactant, what is the maximum mass of iron you can produce?arrow_forwardWhat mass of chlorine gas, in grams, is needed to react completely with 16.3 g of aluminum? 2Al(s)) + 3Cl2(g) → 2AlCl3(s)arrow_forward
- 1) 2 Cu + S> Cużs When copper is heated with sulfur, the reaction above takes place. If 100. g of Cu is heated with 50.0 g of sulfur, which is the limiting reactant? What is the theoretical yield of Cu>S?arrow_forwardConsider the following reaction: If 10.0 g of KF are used, how many grams of CaF2 can be formed? Ca(NO3)2 (aq) + 2 KE (aq) → CaF₂ (s) + 2 KNO3(aq)arrow_forwardBalance the following chemical equation (using the number "1" if the blank normally is left unfilled). Na (s) + NaF (s) F₂ (g) ---->arrow_forward
- Rank the following compounds in order of increasing acidityarrow_forwardA sample of calcium metal with a mass of 2.00 was reacted with excess oxygen. The following equation represents the reaction that took place: 2Ca(s)+O2(g)-> 2CaO(s) the isolated product CaO weighed 2.26g. What was the percent yield of the reaction?arrow_forwardBalance and complete the following combustion reaction. Give the sum (one number only) t e t r a c a r b o n h e x a h y d r i d e ( g ) + o x y g e n ( g ) ⟶ ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY