
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![s[Ni(NH,),] * complex K, -5.50 × 10 at 25 °C.
Suppose equal volumes of 0.0040M Ni (NO₂), solution and 0.26M NH, solution are mixed. Calculate the equilibrium molarity of aqueous Ni²+ ion.
For the aqueous
Round your answer to 2 significant digits.
M](https://content.bartleby.com/qna-images/question/86272b67-3e0f-4818-a4c1-8b50f0248e63/481a253f-f7b6-4c74-8351-65643ec0056f/b4hefje_thumbnail.jpeg)
Transcribed Image Text:s[Ni(NH,),] * complex K, -5.50 × 10 at 25 °C.
Suppose equal volumes of 0.0040M Ni (NO₂), solution and 0.26M NH, solution are mixed. Calculate the equilibrium molarity of aqueous Ni²+ ion.
For the aqueous
Round your answer to 2 significant digits.
M
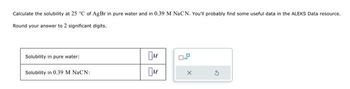
Transcribed Image Text:Calculate the solubility at 25 °C of AgBr in pure water and in 0.39 M NaCN. You'll probably find some useful data in the ALEKS Data resource.
Round your answer to 2 significant digits.
Solubility in pure water:
Solubility in 0.39 M NaCN:
M
M
X
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 15 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- R Please dont provid handwriting solution.arrow_forward4. Consider the solubility product of silver chloride. Which factor(s) will change the numeric value of the equilibrium constant? AgCl(s) Ag*(aq) + C¹ (aq) (i) Addition of Cl¹-(aq); (ii) Removal of Ag*(aq); (iii) Decrease of the pressure; (iv) Change on the temperature of the system. Ksparrow_forward2+ For the aqueous Cu(NH,), 13 complex K, = 2.09 × 10° at 25 °C. 2+ Suppose equal volumes of 0.0020M Cu(NO,), solution and 0.12M NH, solution are mixed. Calculate the equilibrium molarity of aqueous Cu ion. Round your answer to 2 significant digits. 10arrow_forward
- f. Answer the following questions about potassium perchlorate, KCIO,. At 25°C, the value of the solubility product constant, K, for KCIO, (s) is 1.05 X 102. i. Calculate the number of grams of KCIO, (molar mass 138.55 g/mol) that is dissolved in 1000. mL of a saturated solution of KCIO, at 25°C.arrow_forwardConsider the insoluble compound zinc sulfide, ZnS . The zinc ion also forms a complex with hydroxide ions. Write a balanced net ionic equation to show why the solubility of ZnS (s) increases in the presence of hydroxide ions and calculate the equilibrium constant for this reaction. 2.9x10¹5. Be sure to specify states such as (aq) or (s). For Zn(OH)4²-, Kf = K = + Submit Answer + Retry Entire Group 1 more group attempt remainingarrow_forwardPart A A sample of pure NO2 is heated to 334 °C at which temperature it partially dissociates according to the equation 2 NO2 (g) = 2 NO (g) + O2 (g) At equilibrium the density of the gas mixture is 0.520 g/L at 0.750 atm . Calculate K. for the reaction. Π ΑΣφ. K = .0133 Submit Previous Answers Request Answer Incorrect; Try Again; 3 attempts remainingarrow_forward
- When 22.0 mL of a 7.80×10-4 M copper(II) sulfate solution is combined with 25.0 mL of a 5.08×10-4 M sodium carbonate solution does a precipitate form? (yes or no)For these conditions the Reaction Quotient, Q, is equal toarrow_forwardCuBr has a solubility of 2.0 X 10 M at 25 °C. Calculate K for CuBr at 25°C. 1.arrow_forwardFor the aqueous [Hg(NH3)4]2+ complex Kf=1.911019 at 25°C. Suppose equal volumes of 0.0090M HgNO32 solution and 0.36M NH3 solution are mixed. Calculate the equilibrium molarity of aqueous Hg2+ ion. Round your answer to 2 significant digitsarrow_forward
- The equilibrium constant for the dissolution of Mg (OH) 2: Kps = 5.6 x 10-12What is the molar solubility (ies) of Mg (OH) 2 in pure water?arrow_forwardWhen 25.0 mL of a 3.93×10-4 M cobalt(II) bromide solution is combined with 12.0 mL of a 5.61x104 M potassium hydroxide solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal toarrow_forwardWrite the expressions for K, for the following reactions. In each case indicate whether the reaction is homoge- neous or heterogeneous. (a) 203(g) = 302(8) (b) Ti(s) + 2 Cl2(8) = TIC14(I) (c) 2 C,H,(8) + 2H,0(g) = 2 C,H¿(8) + O2(8)| (d) C(s) + 2 H2(8) = CH4(8) (e) 4 HCI(aq) + Oz(8) = 2 H20(1) + 2 Cl2(8) (f) 2 C3H18(1) + 25 O2(g) = 16 CO2(8) + 18 H20(g) (g) 2 C3H18(1) + 25 O2(8) 16 CO2(8) + 18 H20(I)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY