
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For question 3c below, which of the puzzle pieces makes the best sequence for each synthesis? You must solve the syntheses with the puzzle pieces provide and nothing else. One piece can only be used once.
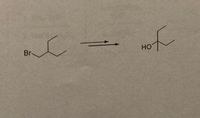
Transcribed Image Text:fou
Br
но
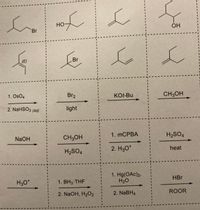
Transcribed Image Text:HO
Br
Br
(E)
1. OsO4
Br2
KOt-Bu
CH3OH
2. NaHSO3 (aq)
light
1. ТСРВА
H2SO4
NaOH
CH3OH
2. H30*
heat
H2SO4
1. Hg(OAc)2,
H2O
HBr
H3O*
1. BH3 THF
2. NaOH, H2O2
2. NABH4
ROOR
3D
3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forward1.Draw a structural formula for the more stable carbocation intermediate formed in the reaction shown. 2.Draw a structural formula for the major organic product of the reaction shown.arrow_forward1A. Explain why solvents such as anhydrous diethyl ether or anhydrous tetrahydrofuran (THF) are not reactive with Grignard reagents. 1B. Draw the first step of the reaction mechanism when the Grignard reagent attacks benzophenone before the alkoxide is protonated to form the alcohol. In the mechanism, identify the partially positive and partially negative atoms in the reactants. Explain why these atoms have a partial charge. 1C. Write the mechanism for the reaction of phenyl magnesium bromide with 1) water and 2) acetone.arrow_forward
- 29 minutes, 42 seconds. Question Completion Status: A Moving to another question will save this response. Question 15 What is not an expected product of the following allylic substitution reaction? NBS, hv Br Br Compounds II and II Compound II only O Compound I only O Compound II only A Moving to another question will save this response O O Carrow_forwardQuestion #9arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY