
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Are the following two reactions valid methods of synthesizing the same organic compound?
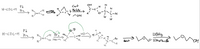
Transcribed Image Text:### Transcription of Reaction Pathways
This diagram illustrates two reaction mechanisms starting from acetylene (H-C≡C-H).
#### Reaction 1:
1. **Hydrogenation Step:**
- Reactant: Acetylene (H-C≡C-H)
- Catalyst: Palladium (Pd)
- Reagent: Hydrogen (H₂)
- Product: Ethylene (H₂C=CH₂)
2. **Epoxidation and Acid-Catalyzed Opening:**
- Reagent: m-CPBA (meta-Chloroperoxybenzoic acid)
- Intermediate: Epoxide forming an oxirane
- Additional Catalysts: Sulfuric acid (H₂SO₄) for ring-opening
- Final Product: 1,2-Ethanediol (OH-CH₂-CH₂-OH)
#### Reaction 2:
1. **Hydrogenation Step:**
- Reactant: Acetylene (H-C≡C-H)
- Catalyst: Palladium (Pd)
- Reagent: Hydrogen (H₂)
- Product: Ethylene (H₂C=CH₂)
2. **Bromination and Formation of Halohydrin:**
- Reagent: Br₂ (Bromine), H₂O (Water)
- Intermediate: Bromonium ion with water opening to form a halohydrin
- Stereochemistry: Attack of the nucleophile (OH⁻) forms the halohydrin
3. **Elimination and Hydroboration-Oxidation:**
- Heat is applied to convert to a cyclic ether
- Reagents: BH₃ for Hydroboration, followed by H₂O₂ and NaOH (sodium hydroxide) for oxidation
- Final Product: 1,2-Ethanediol (CH₃-CH₂-OH)
### Diagram Explanation
- **Flow**: Each pathway follows a series of chemical reactions, depicted with arrows indicating the direction of reactions.
- **Green Curved Arrows**: Indicate electron movement during the formation of intermediate structures in the bromination step.
This educational illustration provides a clear view of multiple reaction pathways beginning with the same starting material, highlighting changes in functional groups and molecular structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- question #31arrow_forwardIn a reaction where a carboxylic ester reacts with water to produce an alcohol and a carboxylate (or carboxylate salt) as the products, the reaction is called ___ , and requires ___ to occur. esterification; strong base saponification; strong base saponification; strong acid acid-catalyzed ester hydrolysis; strong acid esterification; strong acidarrow_forwardquestion #34arrow_forward
- for each of the following reactions labeled (a,b,c,d) write the name of the functional group as well as the correct name for the organic molecule that should appear where the question mark is. You must correctly name the functional group as well as name the structure for each question mark that appears.arrow_forwardWhat is (are) the major organic product(s) obtained from the following substitution reaction? H₂O Br но. 1 OH OH 2 3arrow_forwardQuestion #38arrow_forward
- In an esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic conditions to form an ester. Draw the structure of the ester product in the reaction between pentanoic acid and 1‑propanol. pentanoic acid+ 1-propanol= ester+ H20 structure of ester product neededarrow_forwardQ14 Which statement among the following is incorrect concerning the oxidation or reduction of organic molecules? -Oxidation of alcohols is usually done under acidic or basic conditions to facilitate electron transfer. b- PCC will not oxidize an aldehyde to a carboxylic acid. -All alcohols can be oxidized to form one of an aldehyde, ketone, or carboxylic acid. Reaction of a ketone with a Grignard reagent in ether, followed by dilute acid work-up, produces a tertiary alcohol.arrow_forwardProvide all organic products for the following reaction, including separate geometric isomers if they are formed. DH H2S04 180°C г1 +/- Incorrect 다 +arrow_forward
- Predict the structure of the major organic product that will form when the organic starting material is subjected to the indicated reaction conditions. If no reaction Occurs, indicate that fact in the answer file that you submit. It is not necessary to provide structures or formulas for any inorganic byproducts that also form in the reaction. O,N. 1) Fe, HCI 2) NaOH, H,O 3) NANO , HCI, H,O 4) KI CH3arrow_forwardHow many grams of a 7.50% by mass calcium nitrate solution are needed to obtain 3.75 grams of calcium nitrate?arrow_forwardA chemist hoped to make an ethyl ester using diazoethane. At the end of the reaction, only the original carboxylic acid, ethene, and nitrogen were produced: □ CH H EXP 0+ H₂C OH Suggest an arrow-pushing mechanism that accounts for these products. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. L CONT. 1 H₂C O NEN: OH ΝΕΝ OH *O* + H₂C=CH₂ + N₂ H с N O S CI Br Iarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY