
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
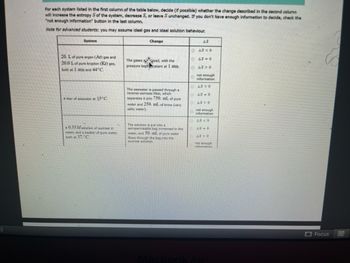
Transcribed Image Text:5)
For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column
will increase the entropy S of the system, decrease S, or leave Sunchanged. If you don't have enough information to decide, check the
"not enough information" button in the last column.
Note for advanced students: you may assume ideal gas and ideal solution behaviour.
Change
System
20. L of pure argon (Ar) gas and
20.0 L of pure krypton (Kr) gas,
both at 1 atm and 44°C.
A liter of seawater an 15°C.
A 0.35 M solution of sucrose in
water, and a beaker of pure water,
both at 37. C
The gases xed, with the
pressure keptstant at 1 atm.
The seawater is passed through a
reverse-osmosis filter, which
separates it into 750. mL of pure
water and 250. mL of brine (very
salty water).
The solution is put into a
semipermeabile bag immersed in the
water, and 50. mL of pure water
flows through the bag into the
sucrose solution.
AS
DAS < 0
AS-0
48>0
not enough
information
AS <0
AS-0
AS O
not enough
information
AS < 0
AS-0
AS 0
not enough
information
MacBook Air
Focus
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. Note for advanced students: you may assume ideal gas and ideal solution behaviour. System A mixture of oxygen (O₂) gas and hydrogen (H₂) gas at 3 atm and 7°C. A liter of seawater at 15°C. 300 mL of a solution made from sodium iodide (Nal) dissolved in water. Change An additional 2.0 L of pure H₂ gas is added to the mixture, with the pressure kept constant at 3 atm. The seawater is passed through a reverse-osmosis filter, which separates it into 750. mL of pure water and 250. mL of brine (very salty water). 0.5 g of Nal crystallizes out of the solution, without changing the temperature. O O AS 0 O As AS 0 not enough information O not enough. information O AS 0 not enough…arrow_forwardes) Qualitatively predicting reaction entropy For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy AS If it's not possible to decide with the information given, check the "not enough information" button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction N₂(g) + 3H₂(g) → 2NH, (e) 4Fe()+30.)- 2Fe₂O, (s) N. (3), (g) 2NH, (g) sign of reaction entropy 04 ron reaction <<0 50 not enough information. 45x 250 <<0 not enough information. 450 50 not enough information. Using the general properties of Gibbs free energy The standard reaction free energy AG" --835. kJ for this reaction: 2 Al(s) + Fe₂O3(s)-Al₂O3(s) + 2 Fe(s) Use this information to complete the table below. Round each of your answers to the nearest kJ. BALAKEN F04 AGO Focusarrow_forwardThe change in entropy, ASxn, is related to the the change in the number of moles of gas molecules, Angas. Determine the change in the moles of gas for each of the reactions and decide if the entropy increases, decreases, or has little or no change. А. 2 H, (g) + O,(g) 2 H,O(1) An gas The entropy, ASixn, mol O has little or no change. O decreases. O increases. В. H,0,(1) H, (g) + O,(g) The entropy, A.SPxn, Angas = mol O increases. O decreases. has little or no change. O Oarrow_forward
- E The Carnot cycle, which is a particular example of a thermodynamic cycle, allows determining the efficiency of a "heat-to-work" engine. Clausius used this to find the macroscopic definition of entropy as the heat change of the system at a particular temperature. Th and T are the high and low qh and q for the heats transferred at these temperatures. When plotted in a T-S temperatures and representation, entropy only changes in the processes (steps) where heat is added or removed. However, when plotting the Carnot cycle in the P-V representation it is clear that work is done (on or by) the system in each of the 4 processes of the cycle. a) Give the names of the 2 processes of the Carnot cycle (an engine) in which the surroundings do work on the system. Indicate the condition(s) of the walls for these processes. b) Consider the microscopic, i.e., Boltzmann's, definition of entropy for an ideal gas. Briefly discuss what needs to be satisfied so that there is no change of entropy during a…arrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. Note for advanced students: you may assume ideal gas and ideal solution behaviour. System Change AS O AS CO A solution made of ammonium bromide (NH Br) in water, at O AS = 0 50. mL of pure water is added to the solution. O AS >0 27°C. not enough information O AS 0 t 27 °C. not enough information O AS 0 argon (Ar) gas at 2 atm and 8°C. pressure kept constant at 2 atm. not enough informationarrow_forwardIf a chemical reaction has a negative reaction entropy, it does not occur spontaneously. Q True Q False Q Search | PSIRITE AIRIT FIF 美美LI 黃 ORIELT O Core 菜单 44 ******** ** ** 24 生事arrow_forward
- a) Formulate the second law of thermodynamics. b) Consider the statements below. Answer them and give brief explanations. a. An explosive (e.g. C7H5N3O6) reacts with oxygen, the result is CO2 (g), H2O (g) and N2 (g). Is there an increase or decrease of Entropy after the reaction occurred? b. Colloquially, people say that during the cold months of the year, the “cold” is creeping into the warmer house. Is this statement true considering the 2nd law?arrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System A few moles of carbon dioxide (CO₂) gas. A few grams of liquid ammonia (NH3). A few moles of carbon dioxide (CO₂) gas. Change The carbon dioxide expands from a volume of 10.0 L to a volume of 13.0 L while the temperature is held constant at 57.0 °C. The ammonia evaporates at a constant temperature of 11.0 °C. The carbon dioxide is cooled from 87.0 °C to -2.0 °C and is also expanded from a volume of 8.0 L to a volume of 11.0 L. AS AS 0 not enough information AS 0 not enough information AS 0 not enough informationarrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. Note for advanced students: you may assume ideal gas and ideal solution behaviour.arrow_forward
- For each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System A few moles of helium (He) gas. A few moles of carbon dioxide (CO₂) gas. A few moles of carbon dioxide (CO₂) gas. Change The helium is compressed from a volume of 11.0 L to a volume of 4.0 L while the temperature is held constant at -6.0 °C. The carbon dioxide is heated from -17.0 °C to 53.0 °C while the volume is held constant at 8.0 L. The carbon dioxide is heated from -8.0 °C to 3.0 °C and also expands from a volume of 10.0 L to a volume of 12.0 L. x AS AS 0 not enough information O AS 0 not enough information O AS 0 not enough informationarrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave Sunchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System A few moles of carbon dioxide (CO₂) gas. A few grams of liquid acetone ((CH₂)₂CO). A few moles of helium (He) gas. Change The carbon dioxide is heated from 5.0 °C to 64.0 °C and is also compressed from a volume of 14.0 L to a volume of 8.0 L. The acetone is heated from -12.0 °C to 89.0 °C. The helium is heated from -14.0 °C to 44.0 °C while the volume is held constant at 9.0 L. X O AS 0 not enough O information O AS 0 not enough information AS 0 O O O O AS O not enough O informationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY