
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
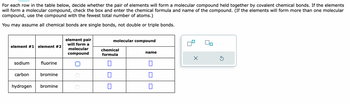
Transcribed Image Text:### Molecular Compound Formation Activity
For each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.)
You may assume all chemical bonds are single bonds, not double or triple bonds.
| **Element #1** | **Element #2** | **Element Pair Will Form a Molecular Compound** | **Molecular Compound** | | |
| -------------- | -------------- | ------------------------------------------------- | ---------------------- | | |
| | | | **Chemical Formula** | **Name** |
| Sodium | Fluorine | ☑ | | |
| Carbon | Bromine | | | |
| Hydrogen | Bromine | ☑ | | |
### Instructions
- **Sodium and Fluorine:** Check the box if a compound can be formed and indicate the chemical formula and name.
- **Carbon and Bromine:** Determine if a molecular compound is possible. If so, add details.
- **Hydrogen and Bromine:** Check the box if applicable, and include the chemical formula and name.
### Interactive Elements
There are options to interact with the activity, such as confirming selections or resetting entries.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they willI, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 compound formed? chemical formula jonic barium bromine O molecular O neither O ionic hydrogen oxygen O molecular O neither O ionic rubidium iodine O molecular neither MAR X P. 27 MacBook Air DD 000 80 11 F9 F10 F7 FB F4 F5 F6 F2 F3 @ $ & 3 4 6 8 9 * COarrow_forward1. Compare cations, anions, and polyatomic ions. What do they all have in common? How are they different? 2. How can the periodic table help to remember the charges on the simple ions of the representative elements? 3. Is there more than one chemical name that can be used for baking soda?arrow_forwardDecide whether each molecule or polyatomic ion is polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. molecule or polyatomic ion F₂ H₂O CHA polar or nonpolar? O polar O nonpolar O polar O nonpolar O polar O nonpolar X atom closest to negative side 0 Sarrow_forward
- I'm having some trouble on my homework and could really use some help See attached:arrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair molecular compound will form a element #1 element #2 molecular chemical name compound formula hydrogen fluorine sodium carbon carbon brominearrow_forwardtable below, decide whether the pair of elements will form a molecu will form a molecular compound, check the box and enter the chemical formula and nam compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 magnesium bromine nitrogen bromine hydrogen fluorine Continue DE element pair will form a molecular compound O 787 molecular compound chemical formula 0 FEB 27 name 0 0 1 X 20 ♫tv Darrow_forward
- What is NOT true about molecular compounds? Select the correct answer below: They are also called covalent compounds. O They are typically formed between nonmetals. They result when atoms transfer electrons to form ions. O They typically exist as gases, low-boiling liquids, and low-melting solids.arrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair will form a alo molecular compound element #1 element #2 molecular chemical name compound formula Ar hydrogen fluorine sodium fluorine nitrogen fluorinearrow_forwardIonic Compounds, Covalent Compounds, and Chemical Equations a. Define the terms "ianic compound" and "covalent compound". Use all of the following terms correctly in at least one of the two definitions cation, anion metal, nonmetol, electrons are shared, and electrons are transferred. Write your answers in complete sentences. b. Complete the following chart using the information given. Correctly write the name or formula of each compound and identify it as lo covalent Formula AlO₂ K₂5 PC1₂ OF Name Magnesium iodide Tetrasulfur tetranitide Iron (III) sulfide Ammonium nitrate Tonico Covalent? CUCO Dinitrogen tetroxide c. Write o balanced chemical equation for the reaction that takes place between magnesium iodide and iron (III) sulfide ond identify the reaction typearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY