
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
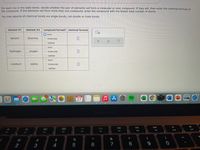
Transcribed Image Text:For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they willI, then enter the chemical formula of
the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms.
You may assume all chemical bonds are single bonds, not double or triple bonds.
element #1
element #2
compound formed? chemical formula
jonic
barium
bromine
O molecular
O neither
O ionic
hydrogen
oxygen
O molecular
O neither
O ionic
rubidium
iodine
O molecular
neither
MAR
X
P.
27
MacBook Air
DD
000
80
11
F9
F10
F7
FB
F4
F5
F6
F2
F3
@
$
&
3
4
6
8
9
* CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe how a covalently bonded molecule is different from compounds that are either ionic or metallic.arrow_forwardGive the total number of valence electrons in each of the following molecules. msp;a.N2Oc.C3H8b.B2H6d.NC13arrow_forwardRepresent the following molecules by Lewis structures: a. CH4 each H atom is bonded to the C atom b. CO2 each O atom is bonded to the C atom c. H2Se each H atom is bonded to the Se atom d. NH3 the H atom is bonded to the N atomarrow_forward
- Write chemical formulas for the following binary molecular compounds. a. Iodine monochloride b. Dinitrogen dioxide c. Nitrogen trichloride d. Hydrogen bromidearrow_forwardFill in the blanks with the smallest integers possible. When gallium (Z=31) reacts with sulfur to form an ionic compound, each metal atom loses ______ electrons and each nonmetal gains_____electronss. There must be _____ gallium atoms for every _____sulfur atoms in the reaction.arrow_forwardThe component elements for four binary molecular compounds are shown with different colors on the following periodic table. What is the chemical formula for the simplest compound likely to be formed in each case? a. Red compound b. Blue compound c. Yellow compound d. Green compoundarrow_forward
- Write chemical formulas for the following binary molecular compounds. a. Bromine monochloride b. Tetrasulfur dinitride c. Sulfur trioxide d. Dioxygen difluoridearrow_forwardWhen potassium and chlorine react and form an ionic compound, why is there only one chlorine atom for each potassium atom instead of two?arrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair alo molecular compound will form a element #1 element #2 molecular chemical name compound formula Ar carbon iodine hydrogen bromine lithium охудеnarrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. molecular compound element #1 element #2 element pair will form a molecular compound chemical name formula magnesium chlorine ☐ ☐ carbon chlorine ☐ ☐ hydrogen iodine ☐ ☐arrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds. element pair will form a alo molecular compound element #1 element #2 molecular chemical name compound formula Ar hydrogen fluorine sodium fluorine nitrogen fluorinearrow_forwardFor each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (If the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning